Denosumab-induced severe hypocalcaemia in a patient with vitamin D deficiency
- PMID: 32847872
- PMCID: PMC7451275
- DOI: 10.1136/bcr-2020-234508
Denosumab-induced severe hypocalcaemia in a patient with vitamin D deficiency
Corrected and republished in
-
Republished: Denosumab-induced severe hypocalcaemia in a patient with vitamin D deficiency.Drug Ther Bull. 2021 Sep;59(9):139-143. doi: 10.1136/dtb.2021.234508rep. Epub 2021 Feb 9. Drug Ther Bull. 2021. PMID: 33563651 No abstract available.
Abstract
Postmenopausal women are at increased risk of osteoporosis. Osteoporotic fractures carry an increased risk of morbidity and mortality. Denosumab is a monoclonal antibody widely used for the treatment of osteoporosis by inhibiting osteoclast-induced bone resorption. Hypocalcaemia is a known side-effect of denosumab treatment. The majority of such cases have been described in patients with underlying metastatic cancer or chronic kidney disease. We present a patient who developed severe hypocalcaemia after administration of denosumab in the context of severe vitamin D deficiency and a normal kidney function. The management was further complicated by hypophosphatemia. Following replacement of vitamin D, the patient's calcium and phosphate levels stabilised. The patient required intensive care monitoring for replacement of electrolytes. This case report emphasises the importance of screening and ongoing monitoring of risk factors for iatrogenic hypocalcaemia with denosumab treatment.
Keywords: calcium and bone; drugs: endocrine system; endocrine system.
© BMJ Publishing Group Limited 2020. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

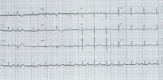

References
-
- McClung MR, Lewiecki EM, Cohen SB, et al. . Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 2006;354:821–31. - PubMed
-
- Bone HG, Bolognese MA, Yuen CK, et al. . Effects of denosumab on bone mineral density and bone turnover in postmenopausal women. J Clin Endocrinol Metab 2008;93:2149–57. - PubMed
-
- Lewiecki EM, Miller PD, McClung MR, et al. . Two-Year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res 2007;22:1832–41. - PubMed
-
- Miller PD, Bolognese MA, Lewiecki EM, et al. . Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 2008;43:222–9. - PubMed
-
- Cummings SR, San Martin J, McClung MR, et al. . Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009;361:756–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
