Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection
- PMID: 32848011
- PMCID: PMC7449630
- DOI: 10.1128/mSphere.00808-20
Development and Clinical Application of a Rapid and Sensitive Loop-Mediated Isothermal Amplification Test for SARS-CoV-2 Infection
Abstract
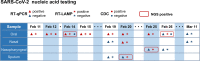
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak urgently necessitates sensitive and convenient COVID-19 diagnostics for the containment and timely treatment of patients. We aimed to develop and validate a novel reverse transcription-loop-mediated isothermal amplification (RT-LAMP) assay to detect SARS-CoV-2. Patients with suspected COVID-19 and close contacts were recruited from two hospitals between 26 January and 8 April 2020. Respiratory samples were collected and tested using RT-LAMP, and the results were compared with those obtained by reverse transcription-quantitative PCR (RT-qPCR). Samples yielding inconsistent results between these two methods were subjected to next-generation sequencing for confirmation. RT-LAMP was also applied to an asymptomatic COVID-19 carrier and patients with other respiratory viral infections. Samples were collected from a cohort of 129 cases (329 nasopharyngeal swabs) and an independent cohort of 76 patients (152 nasopharyngeal swabs and sputum samples). The RT-LAMP assay was validated to be accurate (overall sensitivity and specificity of 88.89% and 99.00%, respectively) and diagnostically useful (positive and negative likelihood ratios of 88.89 and 0.11, respectively). RT-LAMP showed increased sensitivity (88.89% versus 81.48%) and high consistency (kappa, 0.92) compared to those of RT-qPCR for SARS-CoV-2 screening while requiring only constant-temperature heating and visual inspection. The time required for RT-LAMP was less than 1 h from sample preparation to the result. In addition, RT-LAMP was feasible for use with asymptomatic patients and did not cross-react with other respiratory pathogens. The developed RT-LAMP assay offers rapid, sensitive, and straightforward detection of SARS-CoV-2 infection and may aid the expansion of COVID-19 testing in the public domain and hospitals.IMPORTANCE We developed a visual and rapid reverse transcription-loop-mediated isothermal amplification (RT-LAMP) assay targeting the S gene for SARS-CoV-2 infection. The strength of our study was that we validated the RT-LAMP assay using 481 clinical respiratory samples from two prospective cohorts of suspected COVID-19 patients and on the serial samples from an asymptomatic carrier. The developed RT-LAMP approach showed an increased sensitivity (88.89%) and high consistency (kappa, 0.92) compared with those of reverse transcription-quantitative PCR (RT-qPCR) for SARS-CoV-2 screening while requiring only constant-temperature heating and visual inspection, facilitating SARS-CoV-2 screening in well-equipped labs as well as in the field. The time required for RT-LAMP was less than 1 h from sample preparation to the result (more than 2 h for RT-qPCR). This study showed that the RT-LAMP assay was a simple, rapid, and sensitive approach for SARS-CoV-2 infection and can facilitate COVID-19 diagnosis, especially in resource-poor settings.
Keywords: COVID-19; RT-LAMP; SARS-CoV-2; asymptomatic carriers; clinical diagnosis.
Copyright © 2020 Hu et al.
Figures


References
-
- World Health Organization. 2020. Coronavirus disease 2019 (COVID-19) situation report-198. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... Accessed 5 August 2020.
-
- National Health Commission of the People’s Republic of China. 2020. Diagnosis and treatment of novel coronavirus pneumonia (trial version six). http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb19.... (In Chinese.) Accessed 3 March 2020.
-
- World Health Organization. 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. https://www.who.int/docs/default-source/coronaviruse/clinical-management.... Accessed 13 March 2020.
-
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. 2020. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395:565–574. doi:10.1016/S0140-6736(20)30251-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
