The anti-tubercular activity of simvastatin is mediated by cholesterol-driven autophagy via the AMPK-mTORC1-TFEB axis
- PMID: 32848049
- PMCID: PMC7707180
- DOI: 10.1194/jlr.RA120000895
The anti-tubercular activity of simvastatin is mediated by cholesterol-driven autophagy via the AMPK-mTORC1-TFEB axis
Abstract
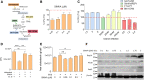
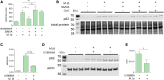
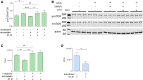
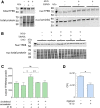
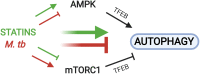
The rise of drug-resistant tuberculosis poses a major risk to public health. Statins, which inhibit both cholesterol biosynthesis and protein prenylation branches of the mevalonate pathway, increase anti-tubercular antibiotic efficacy in animal models. However, the underlying molecular mechanisms are unknown. In this study, we used an in vitro macrophage infection model to investigate simvastatin's anti-tubercular activity by systematically inhibiting each branch of the mevalonate pathway and evaluating the effects of the branch-specific inhibitors on mycobacterial growth. The anti-tubercular activity of simvastatin used at clinically relevant doses specifically targeted the cholesterol biosynthetic branch rather than the prenylation branches of the mevalonate pathway. Using Western blot analysis and AMP/ATP measurements, we found that simvastatin treatment blocked activation of mechanistic target of rapamycin complex 1 (mTORC1), activated AMP-activated protein kinase (AMPK) through increased intracellular AMP:ATP ratios, and favored nuclear translocation of transcription factor EB (TFEB). These mechanisms all induce autophagy, which is anti-mycobacterial. The biological effects of simvastatin on the AMPK-mTORC1-TFEB-autophagy axis were reversed by adding exogenous cholesterol to the cells. Our data demonstrate that the anti-tubercular activity of simvastatin requires inhibiting cholesterol biosynthesis, reveal novel links between cholesterol homeostasis, the AMPK-mTORC1-TFEB axis, and Mycobacterium tuberculosis infection control, and uncover new anti-tubercular therapy targets.
Keywords: Mycobacterium tuberculosis; adenosine 5′-monophosphate-activated protein kinase-mechanistic target of rapamycin complex 1-transcription factor EB axis; immunology; lipids; macrophages/monocytes; mechanistic target of rapamycin complex 1 regulation; statins.
Copyright © 2020 Bruiners et al. Published by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Philips J. A., and Ernst J. D.. 2012. Tuberculosis pathogenesis and immunity. Annu. Rev. Pathol. 7: 353–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

