Hypnotic effect of thalidomide is independent of teratogenic ubiquitin/proteasome pathway
- PMID: 32848052
- PMCID: PMC7502749
- DOI: 10.1073/pnas.1917701117
Hypnotic effect of thalidomide is independent of teratogenic ubiquitin/proteasome pathway
Abstract
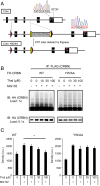
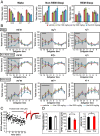
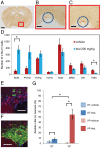
Thalidomide exerts its teratogenic and immunomodulatory effects by binding to cereblon (CRBN) and thereby inhibiting/modifying the CRBN-mediated ubiquitination pathway consisting of the Cullin4-DDB1-ROC1 E3 ligase complex. The mechanism of thalidomide's classical hypnotic effect remains largely unexplored, however. Here we examined whether CRBN is involved in the hypnotic effect of thalidomide by generating mice harboring a thalidomide-resistant mutant allele of Crbn (Crbn YW/AA knock-in mice). Thalidomide increased non-REM sleep time in Crbn YW/AA knock-in homozygotes and heterozygotes to a similar degree as seen in wild-type littermates. Thalidomide similarly depressed excitatory synaptic transmission in the cortical slices obtained from wild-type and Crbn YW/AA homozygous knock-in mice without affecting GABAergic inhibition. Thalidomide induced Fos expression in vasopressin-containing neurons of the supraoptic nucleus and reduced Fos expression in the tuberomammillary nuclei. Thus, thalidomide's hypnotic effect seems to share some downstream mechanisms with general anesthetics and GABAA-activating sedatives but does not involve the teratogenic CRBN-mediated ubiquitin/proteasome pathway.
Keywords: cereblon; electroencephalography/electromyography (EEG/EMG); mouse; sleep; supraoptic nucleus.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide.Leukemia. 2012 Nov;26(11):2326-35. doi: 10.1038/leu.2012.119. Epub 2012 May 3. Leukemia. 2012. PMID: 22552008 Free PMC article.
-
Cereblon E3 ligase complex genes are expressed in tissues sensitive to thalidomide in chicken and zebrafish embryos but are unchanged following thalidomide exposure.Dev Biol. 2025 Jun;522:156-170. doi: 10.1016/j.ydbio.2025.03.014. Epub 2025 Mar 28. Dev Biol. 2025. PMID: 40158790
-
Ligand-mediated protein degradation reveals functional conservation among sequence variants of the CUL4-type E3 ligase substrate receptor cereblon.J Biol Chem. 2018 Apr 20;293(16):6187-6200. doi: 10.1074/jbc.M117.816868. Epub 2018 Feb 15. J Biol Chem. 2018. PMID: 29449372 Free PMC article.
-
[Development of novel cereblon modulators and their target molecules].Rinsho Ketsueki. 2022;63(6):573-579. doi: 10.11406/rinketsu.63.573. Rinsho Ketsueki. 2022. PMID: 35831190 Review. Japanese.
-
The Ubiquitination-Dependent and -Independent Functions of Cereblon in Cancer and Neurological Diseases.J Mol Biol. 2022 Mar 15;434(5):167457. doi: 10.1016/j.jmb.2022.167457. Epub 2022 Jan 16. J Mol Biol. 2022. PMID: 35045330 Review.
Cited by
-
Enhanced homeostatic sleep response and decreased neurodegenerative proteins in cereblon knock-out mice.Commun Biol. 2024 Sep 30;7(1):1218. doi: 10.1038/s42003-024-06879-y. Commun Biol. 2024. PMID: 39349747 Free PMC article.
-
Anti-emetic effects of thalidomide: Evidence, mechanism of action, and future directions.Curr Res Pharmacol Drug Discov. 2022 Oct 27;3:100138. doi: 10.1016/j.crphar.2022.100138. eCollection 2022. Curr Res Pharmacol Drug Discov. 2022. PMID: 36568268 Free PMC article. Review.
-
Identifying c-fos Expression as a Strategy to Investigate the Actions of General Anesthetics on the Central Nervous System.Curr Neuropharmacol. 2022;20(1):55-71. doi: 10.2174/1570159X19666210909150200. Curr Neuropharmacol. 2022. PMID: 34503426 Free PMC article. Review.
-
Human Retinal Organoids in Therapeutic Discovery: A Review of Applications.Handb Exp Pharmacol. 2023;281:157-187. doi: 10.1007/164_2023_691. Handb Exp Pharmacol. 2023. PMID: 37608005 Free PMC article. Review.
-
Predictive biomarkers for embryotoxicity: a machine learning approach to mitigating multicollinearity in RNA-Seq.Arch Toxicol. 2024 Dec;98(12):4093-4105. doi: 10.1007/s00204-024-03852-w. Epub 2024 Sep 6. Arch Toxicol. 2024. PMID: 39242367
References
-
- Taussig H. B., A study of the German outbreak of phocomelia. The thalidomide syndrome. JAMA 180, 1106–1114 (1962). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

