A second X chromosome contributes to resilience in a mouse model of Alzheimer's disease
- PMID: 32848093
- PMCID: PMC8409261
- DOI: 10.1126/scitranslmed.aaz5677
A second X chromosome contributes to resilience in a mouse model of Alzheimer's disease
Abstract
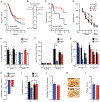
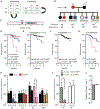
A major sex difference in Alzheimer's disease (AD) is that men with the disease die earlier than do women. In aging and preclinical AD, men also show more cognitive deficits. Here, we show that the X chromosome affects AD-related vulnerability in mice expressing the human amyloid precursor protein (hAPP), a model of AD. XY-hAPP mice genetically modified to develop testicles or ovaries showed worse mortality and deficits than did XX-hAPP mice with either gonad, indicating a sex chromosome effect. To dissect whether the absence of a second X chromosome or the presence of a Y chromosome conferred a disadvantage on male mice, we varied sex chromosome dosage. With or without a Y chromosome, hAPP mice with one X chromosome showed worse mortality and deficits than did those with two X chromosomes. Thus, adding a second X chromosome conferred resilience to XY males and XO females. In addition, the Y chromosome, its sex-determining region Y gene (Sry), or testicular development modified mortality in hAPP mice with one X chromosome such that XY males with testicles survived longer than did XY or XO females with ovaries. Furthermore, a second X chromosome conferred resilience potentially through the candidate gene Kdm6a, which does not undergo X-linked inactivation. In humans, genetic variation in KDM6A was linked to higher brain expression and associated with less cognitive decline in aging and preclinical AD, suggesting its relevance to human brain health. Our study suggests a potential role for sex chromosomes in modulating disease vulnerability related to AD.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures






Similar articles
-
Four core genotypes mouse model: localization of the Sry transgene and bioassay for testicular hormone levels.BMC Res Notes. 2015 Mar 7;8:69. doi: 10.1186/s13104-015-0986-2. BMC Res Notes. 2015. PMID: 25870930 Free PMC article.
-
Evidence that the testis determination pathway interacts with a non-dosage compensated, X-linked gene.Int J Dev Biol. 2001;45(3):509-12. Int J Dev Biol. 2001. PMID: 11417892
-
X, but not Y, Chromosomal Complement Contributes to Stroke Sensitivity in Aged Animals.Transl Stroke Res. 2023 Oct;14(5):776-789. doi: 10.1007/s12975-022-01070-z. Epub 2022 Jul 29. Transl Stroke Res. 2023. PMID: 35906327 Free PMC article.
-
Sex determination and sex reversal: genotype, phenotype, dogma and semantics.Hum Genet. 1992 Jul;89(5):467-79. doi: 10.1007/BF00219168. Hum Genet. 1992. PMID: 1634224 Review.
-
Aberrant chromosomal sex-determining mechanisms in mammals, with special reference to species with XY females.Philos Trans R Soc Lond B Biol Sci. 1988 Dec 1;322(1208):83-95. doi: 10.1098/rstb.1988.0116. Philos Trans R Soc Lond B Biol Sci. 1988. PMID: 2907806 Review.
Cited by
-
Cognitive Resilience in Brain Health and Dementia Research.J Alzheimers Dis. 2022;90(2):461-473. doi: 10.3233/JAD-220755. J Alzheimers Dis. 2022. PMID: 36093713 Free PMC article. Review.
-
Loss of Y chromosome at the interface between aging and Alzheimer's disease.Cell Mol Life Sci. 2021 Nov;78(21-22):7081-7084. doi: 10.1007/s00018-021-03935-2. Epub 2021 Sep 9. Cell Mol Life Sci. 2021. PMID: 34498098 Free PMC article. No abstract available.
-
The maternal X chromosome affects cognition and brain ageing in female mice.Nature. 2025 Feb;638(8049):152-159. doi: 10.1038/s41586-024-08457-y. Epub 2025 Jan 22. Nature. 2025. PMID: 39843739 Free PMC article.
-
The Four Core Genotypes mouse model: evaluating the impact of a recently discovered translocation.Biol Sex Differ. 2024 Oct 31;15(1):90. doi: 10.1186/s13293-024-00665-5. Biol Sex Differ. 2024. PMID: 39482704 Free PMC article.
-
Insights into Sex and Gender Differences in Brain and Psychopathologies Using Big Data.Life (Basel). 2023 Aug 2;13(8):1676. doi: 10.3390/life13081676. Life (Basel). 2023. PMID: 37629533 Free PMC article. Review.
References
-
- McCarthy MM, Woolley CS, Arnold AP, Incorporating sex as a biological variable in neuroscience: What do we gain? Nat. Rev. Neurosci 18, 707–708 (2017). - PubMed
-
- Prince M, Wimo A, Guerchet M, Ali G-C, Wu Y-T, Prina M, World Alzheimer Report 2015: The Global Impact of Dementia (Alzheimer’s Disease International (ADI), 2015).
-
- Dubal DB, Sex Differences in Alzheimer’s disease: An updated, balanced, and emerging perspective on differing vulnerabilities, in Sex Differences in Neurology and Psychiatry, Lanzberger R, Kranz GS, Savic-Berglund I, Eds., Handbook of Clinical Neurology, Vol. 175 (3rd series) (Elsevier, 2020); 10.1016/B978-0-444-64123-6.00018-7. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS043196/NS/NINDS NIH HHS/United States
- R01 AG011385/AG/NIA NIH HHS/United States
- K01 AG049152/AG/NIA NIH HHS/United States
- R01 AG062588/AG/NIA NIH HHS/United States
- U01 AG061356/AG/NIA NIH HHS/United States
- U01 AG024904/AG/NIA NIH HHS/United States
- R01 HD076125/HD/NICHD NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- P30 NS065780/NS/NINDS NIH HHS/United States
- RF1 AG068325/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- K08 AG034531/AG/NIA NIH HHS/United States
- R01 GM128431/GM/NIGMS NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- R01 AG062629/AG/NIA NIH HHS/United States
- U01 AG046152/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- R37 AG011385/AG/NIA NIH HHS/United States
- R01 AG036836/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
- RF1 AG062234/AG/NIA NIH HHS/United States
- R01 NS092918/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

