Tubeimoside I-induced lung cancer cell death and the underlying crosstalk between lysosomes and mitochondria
- PMID: 32848130
- PMCID: PMC7449972
- DOI: 10.1038/s41419-020-02915-x
Tubeimoside I-induced lung cancer cell death and the underlying crosstalk between lysosomes and mitochondria
Abstract
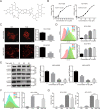
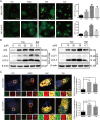
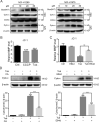
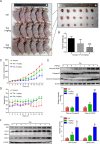
Cancer cells have developed chemoresistance and have improved their survival through the upregulation of autophagic mechanisms that protect mitochondrial function. Here, we report that the traditional Chinese anticancer agent tubeimoside I (Tub), which is a potent inhibitor of autophagy, can promote mitochondria-associated apoptosis in lung cancer cells. We found that Tub disrupted both mitochondrial and lysosomal pathways. One of its mechanisms was the induction of DRP1-mediated mitochondrial fragmentation. Another mechanism was the blocking of late-stage autophagic flux via impairment of lysosomal acidification through V-ATPase inhibition; this blocks the removal of dysfunctional mitochondria and results in reactive oxygen species (ROS) accumulation. Excessive ROS accumulation causes damage to lysosomal membranes and increases lysosomal membrane permeability, which leads to the leakage of cathepsin B. Finally, cathepsin B upregulates Bax-mediated mitochondrial outer membrane permeability and, subsequently, cytosolic cytochrome C-mediated caspase-dependent apoptosis. Thus, the cancer cell killing effect of Tub is enhanced through the formation of a positive feedback loop. The killing effect of Tub on lung cancer cells was verified in xenografted mice. In summary, Tub exerts a dual anticancer effect that involves the disruption of mitochondrial and lysosomal pathways and their interaction and, thereby, has a specific and enhanced killing effect on lung cancer cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Lysosomal and mitochondrial permeabilization mediates zinc(II) cationic phthalocyanine phototoxicity.Int J Biochem Cell Biol. 2013 Nov;45(11):2553-62. doi: 10.1016/j.biocel.2013.08.012. Epub 2013 Aug 28. Int J Biochem Cell Biol. 2013. PMID: 23994488
-
Identification of compound CA-5f as a novel late-stage autophagy inhibitor with potent anti-tumor effect against non-small cell lung cancer.Autophagy. 2019 Mar;15(3):391-406. doi: 10.1080/15548627.2018.1511503. Epub 2018 Sep 6. Autophagy. 2019. PMID: 30145925 Free PMC article.
-
Tubeimoside I induces accumulation of impaired autophagolysosome against cervical cancer cells by both initiating autophagy and inhibiting lysosomal function.Cell Death Dis. 2018 Nov 2;9(11):1117. doi: 10.1038/s41419-018-1151-3. Cell Death Dis. 2018. PMID: 30389907 Free PMC article.
-
Host defense peptides as new weapons in cancer treatment.Cell Mol Life Sci. 2005 Apr;62(7-8):784-90. doi: 10.1007/s00018-005-4560-2. Cell Mol Life Sci. 2005. PMID: 15868403 Free PMC article. Review.
-
Natural Products Induce Lysosomal Membrane Permeabilization as an Anticancer Strategy.Medicines (Basel). 2021 Nov 10;8(11):69. doi: 10.3390/medicines8110069. Medicines (Basel). 2021. PMID: 34822366 Free PMC article. Review.
Cited by
-
Polyploid giant cancer cells: origin, possible pathways of formation, characteristics, and mechanisms of regulation.Front Cell Dev Biol. 2024 Jul 11;12:1410637. doi: 10.3389/fcell.2024.1410637. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39055650 Free PMC article. Review.
-
Saponin Fraction CIL1 from Lysimachia ciliata L. Enhances the Effect of a Targeted Toxin on Cancer Cells.Pharmaceutics. 2023 Apr 28;15(5):1350. doi: 10.3390/pharmaceutics15051350. Pharmaceutics. 2023. PMID: 37242592 Free PMC article.
-
Metabolic Dysregulation and Neurovascular Dysfunction in Diabetic Retinopathy.Antioxidants (Basel). 2020 Dec 8;9(12):1244. doi: 10.3390/antiox9121244. Antioxidants (Basel). 2020. PMID: 33302369 Free PMC article. Review.
-
Novel Tubeimoside I liposomal drug delivery system in combination with gemcitabine for the treatment of pancreatic cancer.Nanomedicine (Lond). 2024;19(24):1977-1993. doi: 10.1080/17435889.2024.2382076. Epub 2024 Sep 3. Nanomedicine (Lond). 2024. PMID: 39225145 Free PMC article.
-
Tubeimoside-1 Enhances TRAIL-Induced Apoptotic Cell Death through STAMBPL1-Mediated c-FLIP Downregulation.Int J Mol Sci. 2023 Jul 24;24(14):11840. doi: 10.3390/ijms241411840. Int J Mol Sci. 2023. PMID: 37511599 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

