Antimicrobial Property of Halogenated Catechols
- PMID: 32848507
- PMCID: PMC7444726
- DOI: 10.1016/j.cej.2020.126340
Antimicrobial Property of Halogenated Catechols
Abstract
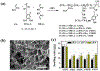
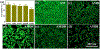
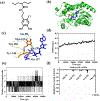
Bacterial infection associated with multidrug resistance (MDR) bacteria is increasingly becoming a significant public health risk. Herein, we synthesized a series of halogenated dopamine methacrylamide (DMA), which contains a catechol side chain modified with either chloro-, bromo-, or iodo-functional group. Catechol is a widely used adhesive moiety for designing bioadhesives and coating. However, the intrinsic antimicrobial property of catechol has not been demonstrated before. These halogenated DMA were incorporated into hydrogels, copolymers, and coatings and exhibited more than 99% killing efficiencies against Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli. More importantly, hydrogel containing chlorinated DMA demonstrated broad-spectrum antimicrobial activities towards multiple MDR bacteria, which included methicillin resistant S. aureus (MRSA), vancomycin resistant enterococci (VRE), multi antibiotics resistant Pseudomonas aeruginosa (PAER), multi antibiotics resistant Acinetobacter baumannii (AB) and carbapenem resistant Klebsiella pneumoniae (CRKP). These hydrogels also demonstrated the ability to kill bacteria in a biofilm while exhibiting low cytotoxic. Based on molecular docking and molecular dynamics simulation, Cl-functionalized catechol can potentially inhibit bacterial fatty acid synthesis at the enoyl-acyl carrier protein reductase (FabI) step. The combination of moisture-resistant adhesive property, inherent antimicrobial property, and the versatility of incorporating halogenated DMA into different polymeric materials greatly enhanced the potential for using these monomers for designing multifunctional bioadhesives and coatings.
Keywords: antimicrobial; dopamine methacrylamide; halogenated catechol; multidrug resistant bacteria; mussel adhesive proteins.
Conflict of interest statement
Declaration of interests The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



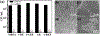

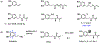
Similar articles
-
Patterns of Drug-Resistant Bacteria in a General Hospital, China, 2011-2016.Pol J Microbiol. 2019;68(2):225-232. doi: 10.33073/pjm-2019-024. Pol J Microbiol. 2019. PMID: 31250593 Free PMC article.
-
pH Responsive Antibacterial Hydrogel Utilizing Catechol-Boronate Complexation Chemistry.Chem Eng J. 2022 Aug 1;441:135808. doi: 10.1016/j.cej.2022.135808. Epub 2022 Mar 16. Chem Eng J. 2022. PMID: 35444488 Free PMC article.
-
The tigecycline evaluation and surveillance trial; assessment of the activity of tigecycline and other selected antibiotics against gram-positive and gram-negative pathogens from France collected between 2004 and 2016.Antimicrob Resist Infect Control. 2018 May 30;7:68. doi: 10.1186/s13756-018-0360-y. eCollection 2018. Antimicrob Resist Infect Control. 2018. PMID: 29876099 Free PMC article.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
Cited by
-
A Facile Surface Modification Scheme for Medical-Grade Titanium and Polypropylene Using a Novel Mussel-Inspired Biomimetic Polymer with Cationic Quaternary Ammonium Functionalities for Antibacterial Application.Polymers (Basel). 2024 Feb 12;16(4):503. doi: 10.3390/polym16040503. Polymers (Basel). 2024. PMID: 38399881 Free PMC article.
-
Catechol-Based Antimicrobial Polymers.Molecules. 2021 Jan 21;26(3):559. doi: 10.3390/molecules26030559. Molecules. 2021. PMID: 33494541 Free PMC article. Review.
-
Design of multiple-function matrix encapsulated with Marjoram extract to support cellular functions, stimulate collagen synthesis and decrease infection in wound.Sci Rep. 2024 Sep 10;14(1):21109. doi: 10.1038/s41598-024-71525-w. Sci Rep. 2024. PMID: 39256491 Free PMC article.
-
Polymeric Composite Thin Films Deposited by Laser Techniques for Antimicrobial Applications-A Short Overview.Polymers (Basel). 2025 Jul 24;17(15):2020. doi: 10.3390/polym17152020. Polymers (Basel). 2025. PMID: 40808071 Free PMC article. Review.
-
Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens.Front Pharmacol. 2021 Jul 21;12:720726. doi: 10.3389/fphar.2021.720726. eCollection 2021. Front Pharmacol. 2021. PMID: 34366872 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
