Minocycline Treatment Reverses Sound Evoked EEG Abnormalities in a Mouse Model of Fragile X Syndrome
- PMID: 32848552
- PMCID: PMC7417521
- DOI: 10.3389/fnins.2020.00771
Minocycline Treatment Reverses Sound Evoked EEG Abnormalities in a Mouse Model of Fragile X Syndrome
Abstract
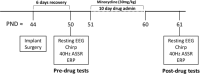
Fragile X Syndrome (FXS) is a leading known genetic cause of intellectual disability. Many symptoms of FXS overlap with those in autism including repetitive behaviors, language delays, anxiety, social impairments and sensory processing deficits. Electroencephalogram (EEG) recordings from humans with FXS and an animal model, the Fmr1 knockout (KO) mouse, show remarkably similar phenotypes suggesting that EEG phenotypes can serve as biomarkers for developing treatments. This includes enhanced resting gamma band power and sound evoked total power, and reduced fidelity of temporal processing and habituation of responses to repeated sounds. Given the therapeutic potential of the antibiotic minocycline in humans with FXS and animal models, it is important to determine sensitivity and selectivity of EEG responses to minocycline. Therefore, in this study, we examined if a 10-day treatment of adult Fmr1 KO mice with minocycline (oral gavage, 30 mg/kg per day) would reduce EEG abnormalities. We tested if minocycline treatment has specific effects based on the EEG measurement type (e.g., resting versus sound-evoked) from the frontal and auditory cortex of the Fmr1 KO mice. We show increased resting EEG gamma power and reduced phase locking to time varying stimuli as well as the 40 Hz auditory steady state response in the Fmr1 KO mice in the pre-drug condition. Minocycline treatment increased gamma band phase locking in response to auditory stimuli, and reduced sound-evoked power of auditory event related potentials (ERP) in Fmr1 KO mice compared to vehicle treatment. Minocycline reduced resting EEG gamma power in Fmr1 KO mice, but this effect was similar to vehicle treatment. We also report frequency band-specific effects on EEG responses. Taken together, these data indicate that sound-evoked EEG responses may serve as more sensitive measures, compared to resting EEG measures, to isolate minocycline effects from placebo in humans with FXS. Given the use of minocycline and EEG recordings in a number of neurodegenerative and neurodevelopmental conditions, these findings may be more broadly applicable in translational neuroscience.
Keywords: EEG; MMP-9; autism; forebrain; fragile X syndrome; minocycline; sensory hypersensitivity.
Copyright © 2020 Lovelace, Ethell, Binder and Razak.
Figures





References
-
- Babiloni C., Infarinato F., Aujard F., Bastlund J. F., Bentivoglio M., Bertini G., et al. (2013). Effects of pharmacological agents, sleep deprivation, hypoxia and transcranial magnetic stimulation on electroencephalographic rhythms in rodents: towards translational challenge models for drug discovery in Alzheimer’s disease. Clin. Neurophysiol. 124 437–451. 10.1016/j.clinph.2012.07.023 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

