TRP Channels Role in Pain Associated With Neurodegenerative Diseases
- PMID: 32848557
- PMCID: PMC7417429
- DOI: 10.3389/fnins.2020.00782
TRP Channels Role in Pain Associated With Neurodegenerative Diseases
Abstract
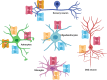
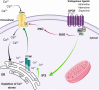
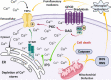
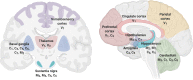
Transient receptor potential (TRP) are cation channels expressed in both non-excitable and excitable cells from diverse tissues, including heart, lung, and brain. The TRP channel family includes 28 isoforms activated by physical and chemical stimuli, such as temperature, pH, osmotic pressure, and noxious stimuli. Recently, it has been shown that TRP channels are also directly or indirectly activated by reactive oxygen species. Oxidative stress plays an essential role in neurodegenerative disorders, such as Alzheimer's and Parkinson's diseases, and TRP channels are involved in the progression of those diseases by mechanisms involving changes in the crosstalk between Ca2+ regulation, oxidative stress, and production of inflammatory mediators. TRP channels involved in nociception include members of the TRPV, TRPM, TRPA, and TRPC subfamilies that transduce physical and chemical noxious stimuli. It has also been reported that pain is a complex issue in patients with Alzheimer's and Parkinson's diseases, and adequate management of pain in those conditions is still in discussion. TRPV1 has a role in neuroinflammation, a critical mechanism involved in neurodegeneration. Therefore, some studies have considered TRPV1 as a target for both pain treatment and neurodegenerative disorders. Thus, this review aimed to describe the TRP-dependent mechanism that can mediate pain sensation in neurodegenerative diseases and the therapeutic approach available to palliate pain and neurodegenerative symptoms throughout the regulation of these channels.
Keywords: Alzheimer’s disease; Parkinson’s disease; TRP channels; neurodegeneration; pain.
Copyright © 2020 Duitama, Vargas-López, Casas, Albarracin, Sutachan and Torres.
Figures
Similar articles
-
TRP Channels and Pain.In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 8. In: Emir TLR, editor. Neurobiology of TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2017. Chapter 8. PMID: 29356491 Free Books & Documents. Review.
-
TRP channels: Role in neurodegenerative diseases and therapeutic targets.Heliyon. 2023 Jun 2;9(6):e16910. doi: 10.1016/j.heliyon.2023.e16910. eCollection 2023 Jun. Heliyon. 2023. PMID: 37332910 Free PMC article. Review.
-
TRP Channels in Nociception and Pathological Pain.Adv Exp Med Biol. 2018;1099:13-27. doi: 10.1007/978-981-13-1756-9_2. Adv Exp Med Biol. 2018. PMID: 30306511 Review.
-
High-Throughput Approaches to Studying Mechanisms of TRP Channel Activation.In: Zhu MX, editor. TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. Chapter 12. In: Zhu MX, editor. TRP Channels. Boca Raton (FL): CRC Press/Taylor & Francis; 2011. Chapter 12. PMID: 22593966 Free Books & Documents. Review.
-
Downregulation of TRPM7, TRPM8, and TRPV1 channels modulate apoptotic parameters and neurodegenerative markers: Focus on neuronal differentiation and Parkinson's disease model.Cell Biol Int. 2023 Sep;47(9):1502-1518. doi: 10.1002/cbin.12048. Epub 2023 May 19. Cell Biol Int. 2023. PMID: 37208975
Cited by
-
Progress in the Structural Basis of thermoTRP Channel Polymodal Gating.Int J Mol Sci. 2023 Jan 1;24(1):743. doi: 10.3390/ijms24010743. Int J Mol Sci. 2023. PMID: 36614186 Free PMC article. Review.
-
The metabolic and functional roles of sensory nerves in adipose tissues.Nat Metab. 2023 Sep;5(9):1461-1474. doi: 10.1038/s42255-023-00868-x. Epub 2023 Sep 14. Nat Metab. 2023. PMID: 37709960 Review.
-
Molecular Mechanisms of Neurogenic Inflammation of the Skin.Int J Mol Sci. 2023 Mar 5;24(5):5001. doi: 10.3390/ijms24055001. Int J Mol Sci. 2023. PMID: 36902434 Free PMC article. Review.
-
TRPA1 Role in Inflammatory Disorders: What Is Known So Far?Int J Mol Sci. 2022 Apr 20;23(9):4529. doi: 10.3390/ijms23094529. Int J Mol Sci. 2022. PMID: 35562920 Free PMC article. Review.
-
Manmade Electromagnetic Fields and Oxidative Stress-Biological Effects and Consequences for Health.Int J Mol Sci. 2021 Apr 6;22(7):3772. doi: 10.3390/ijms22073772. Int J Mol Sci. 2021. PMID: 33917298 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

