Collapsin Response Mediator Protein 2 and Endophilin2 Coordinate Regulation of AMPA Receptor GluA1 Subunit Recycling
- PMID: 32848595
- PMCID: PMC7417516
- DOI: 10.3389/fnmol.2020.00128
Collapsin Response Mediator Protein 2 and Endophilin2 Coordinate Regulation of AMPA Receptor GluA1 Subunit Recycling
Abstract
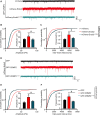
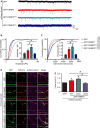
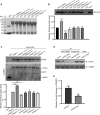
The dynamic trafficking of AMPA receptors (AMPARs), which enables the endocytosis, recycling, and exocytosis of AMPARs, is crucial for synaptic plasticity. Endophilin2, which directly interacts with the GluA1 subunit of AMPARs, plays an important role in AMPAR endocytosis. Collapsin response mediator protein 2 (CRMP2) promotes the maturation of the dendritic spine and can transfer AMPARs to the membrane. Although the mechanisms of AMPAR endocytosis and exocytosis are well known, the exact molecular mechanisms underlying AMPAR recycling remain unclear. Here, we report a unique interaction between CRMP2 and endophilin2. Our results showed that overexpression of CRMP2 and endophilin2 increased the amplitude and frequency of miniature excitatory synaptic currents (mEPSCs) and modestly enhanced AMPAR levels in hippocampal neurons. Furthermore, the CRMP2 and endophilin2 overexpression phenotype failed to occur when the interaction between these two proteins was inhibited. Further analysis revealed that this interaction was regulated by CRMP2 phosphorylation. The phosphorylation of CRMP2 inhibited its interaction with endophilin2; this was mainly affected by the phosphorylation of Thr514 and Ser518 by glycogen synthase kinase (GSK) 3β. CRMP2 phosphorylation increased degradation and inhibited the surface expression of AMPAR GluA1 subunits in cultured hippocampal neurons. However, the dephosphorylation of CRMP2 inhibited degradation and promoted the surface expression of AMPAR GluA1 subunits in cultured hippocampal neurons. Taken together, our data demonstrated that the interaction between CRMP2 and endophilin2 was conductive to the recycling of AMPA receptor GluA1 subunits in hippocampal neurons.
Keywords: AMPA receptor; GluA1 subunit; collapsing response mediator protein 2; endophilin2; recycling.
Copyright © 2020 Zhang, Li, Yin, Li, Jiang, Wang Cha and Guo.
Figures





Similar articles
-
Phosphorylation of CRMP2 by Cdk5 Negatively Regulates the Surface Delivery and Synaptic Function of AMPA Receptors.Mol Neurobiol. 2022 Feb;59(2):762-777. doi: 10.1007/s12035-021-02581-w. Epub 2021 Nov 12. Mol Neurobiol. 2022. PMID: 34773219
-
Endophilin2 Interacts with GluA1 to Mediate AMPA Receptor Endocytosis Induced by Oligomeric Amyloid-β.Neural Plast. 2017;2017:8197085. doi: 10.1155/2017/8197085. Epub 2017 Jul 5. Neural Plast. 2017. PMID: 28758034 Free PMC article.
-
Collapsin response mediator protein 2 modulates synaptic function by stabilizing the surface expression of phosphorylated AMPA receptor GluA2 subunits.Brain Res. 2025 Sep 15;1863:149798. doi: 10.1016/j.brainres.2025.149798. Epub 2025 Jul 5. Brain Res. 2025. PMID: 40618868
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
-
Regulation of AMPA Receptor Trafficking by Protein Ubiquitination.Front Mol Neurosci. 2017 Oct 26;10:347. doi: 10.3389/fnmol.2017.00347. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29123470 Free PMC article. Review.
Cited by
-
Phosphorylation of Spastin Promotes the Surface Delivery and Synaptic Function of AMPA Receptors.Front Cell Neurosci. 2022 Mar 28;16:809934. doi: 10.3389/fncel.2022.809934. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35418834 Free PMC article.
-
Autophagy regulates neuronal excitability by controlling cAMP/protein kinase A signaling at the synapse.EMBO J. 2022 Nov 17;41(22):e110963. doi: 10.15252/embj.2022110963. Epub 2022 Oct 11. EMBO J. 2022. PMID: 36217825 Free PMC article.
-
Phosphorylation of CRMP2 by Cdk5 Negatively Regulates the Surface Delivery and Synaptic Function of AMPA Receptors.Mol Neurobiol. 2022 Feb;59(2):762-777. doi: 10.1007/s12035-021-02581-w. Epub 2021 Nov 12. Mol Neurobiol. 2022. PMID: 34773219
-
Cationic peptides erase memories by removing synaptic AMPA receptors through endophilin-mediated endocytosis.Res Sq [Preprint]. 2023 Nov 21:rs.3.rs-3559525. doi: 10.21203/rs.3.rs-3559525/v1. Res Sq. 2023. Update in: Nature. 2025 Feb;638(8050):479-489. doi: 10.1038/s41586-024-08413-w. PMID: 38045269 Free PMC article. Updated. Preprint.
-
Effects of DeSUMOylated Spastin on AMPA Receptor Surface Delivery and Synaptic Function Are Enhanced by Phosphorylating at Ser210.Mol Neurobiol. 2024 Aug;61(8):6045-6059. doi: 10.1007/s12035-024-03935-w. Epub 2024 Jan 24. Mol Neurobiol. 2024. PMID: 38267753
References
-
- Arimura N., Inagaki N., Chihara K., Ménager C., Nakamura N., Amano M., et al. . (2000). Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J. Biol. Chem. 275, 23973–23980. 10.1074/jbc.m001032200 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

