Role of Chondroitin Sulfation Following Spinal Cord Injury
- PMID: 32848612
- PMCID: PMC7419623
- DOI: 10.3389/fncel.2020.00208
Role of Chondroitin Sulfation Following Spinal Cord Injury
Abstract
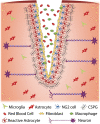
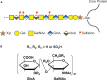
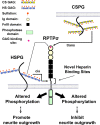
Traumatic spinal cord injury produces long-term neurological damage, and presents a significant public health problem with nearly 18,000 new cases per year in the U.S. The injury results in both acute and chronic changes in the spinal cord, ultimately resulting in the production of a glial scar, consisting of multiple cells including fibroblasts, macrophages, microglia, and reactive astrocytes. Within the scar, there is an accumulation of extracellular matrix (ECM) molecules-primarily tenascins and chondroitin sulfate proteoglycans (CSPGs)-which are considered to be inhibitory to axonal regeneration. In this review article, we discuss the role of CSPGs in the injury response, especially how sulfated glycosaminoglycan (GAG) chains act to inhibit plasticity and regeneration. This includes how sulfation of GAG chains influences their biological activity and interactions with potential receptors. Comprehending the role of CSPGs in the inhibitory properties of the glial scar provides critical knowledge in the much-needed production of new therapies.
Keywords: axon guidance; glial scar; glycosaminoglycan; proteoglycan; receptor tyrosine phosphatase.
Copyright © 2020 Hussein, Mencio, Katagiri, Brake and Geller.
Figures
Similar articles
-
Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans.Exp Neurol. 2015 Dec;274(Pt B):115-25. doi: 10.1016/j.expneurol.2015.08.015. Epub 2015 Aug 24. Exp Neurol. 2015. PMID: 26315937 Free PMC article.
-
Chondroitin sulfate proteoglycans: Key modulators in the developing and pathologic central nervous system.Exp Neurol. 2015 Jul;269:169-87. doi: 10.1016/j.expneurol.2015.04.006. Epub 2015 Apr 18. Exp Neurol. 2015. PMID: 25900055 Review.
-
Modulation of Receptor Protein Tyrosine Phosphatase Sigma Increases Chondroitin Sulfate Proteoglycan Degradation through Cathepsin B Secretion to Enhance Axon Outgrowth.J Neurosci. 2018 Jun 6;38(23):5399-5414. doi: 10.1523/JNEUROSCI.3214-17.2018. Epub 2018 May 14. J Neurosci. 2018. PMID: 29760175 Free PMC article.
-
Chondroitin sulfate proteoglycan 4,6 sulfation regulates sympathetic nerve regeneration after myocardial infarction.Elife. 2022 May 23;11:e78387. doi: 10.7554/eLife.78387. Elife. 2022. PMID: 35604022 Free PMC article.
-
NG2/CSPG4 and progranulin in the posttraumatic glial scar.Matrix Biol. 2018 Aug;68-69:571-588. doi: 10.1016/j.matbio.2017.10.002. Epub 2017 Oct 17. Matrix Biol. 2018. PMID: 29054751 Review.
Cited by
-
The role of nitric oxide and hydrogen sulfide in spinal cord injury: an updated review.Med Gas Res. 2024 Sep 1;14(3):96-101. doi: 10.4103/2045-9912.385946. Epub 2023 Sep 17. Med Gas Res. 2024. PMID: 39073336 Free PMC article. Review.
-
Spatiotemporal diversity and regulation of glycosaminoglycans in cell homeostasis and human disease.Am J Physiol Cell Physiol. 2022 May 1;322(5):C849-C864. doi: 10.1152/ajpcell.00085.2022. Epub 2022 Mar 16. Am J Physiol Cell Physiol. 2022. PMID: 35294848 Free PMC article. Review.
-
Sustained delivery of chABC improves functional recovery after a spine injury.BMC Neurosci. 2022 Oct 28;23(1):60. doi: 10.1186/s12868-022-00734-8. BMC Neurosci. 2022. PMID: 36307768 Free PMC article.
-
Ischemic stroke disrupts the endothelial glycocalyx through activation of proHPSE via acrolein exposure.J Biol Chem. 2020 Dec 25;295(52):18614-18624. doi: 10.1074/jbc.RA120.015105. Epub 2020 Oct 30. J Biol Chem. 2020. PMID: 33127645 Free PMC article.
-
Scientific Advances in Neural Regeneration After Spinal Cord Injury.Cureus. 2025 Feb 6;17(2):e78630. doi: 10.7759/cureus.78630. eCollection 2025 Feb. Cureus. 2025. PMID: 40062077 Free PMC article. Review.
References
-
- Bao X., Nishimura S., Mikami T., Yamada S., Itoh N., Sugahara K. (2004). Chondroitin sulfate/dermatan sulfate hybrid chains from embryonic pig brain, which contain a higher proportion of L-iduronic acid than those from adult pig brain, exhibit neuritogenic and growth factor binding activities. J. Biol. Chem. 279, 9765–9776. 10.1074/jbc.m310877200 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

