Substantia Nigra Hyperechogenicity Reflects the Progression of Dopaminergic Neurodegeneration in 6-OHDA Rat Model of Parkinson's Disease
- PMID: 32848616
- PMCID: PMC7418516
- DOI: 10.3389/fncel.2020.00216
Substantia Nigra Hyperechogenicity Reflects the Progression of Dopaminergic Neurodegeneration in 6-OHDA Rat Model of Parkinson's Disease
Abstract
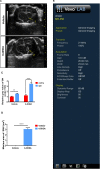
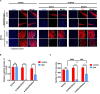
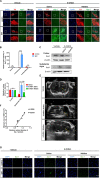
Parkinson's disease (PD) is the second most common neurodegenerative disease, and there is still no effective way to stop its progress. Therefore, early detection is crucial for the prevention and the treatment of Parkinson's disease. The current diagnosis of Parkinson's disease, however, mainly depends on the symptoms, so it is necessary to establish a reliable imaging modality for PD diagnosis and its progression monitoring. Other studies and our previous ones demonstrated that substantia nigra hyperechogenicity (SNH) was detected by transcranial sonography (TCS) in the ventral midbrain of PD patients, and SNH is regarded as a characteristic marker of PD. The present study aimed to explore whether SNH could serve as a reliable imaging modality to monitor the progression of dopaminergic neurodegeneration of PD. The results revealed that the size of SNH was positively related with the degree of dopaminergic neuron death in PD animal models. Furthermore, we revealed that microglia activation contributed to the SNH formation in substantia nigra (SN) in PD models. Taken together, this study suggests that SNH through TCS is a promising imaging modality to monitor the progression of dopaminergic neurodegeneration of PD.
Keywords: Parkinson’s disease; dopaminergic neuron; microglia activation; substantia nigra hyperechogenicity; transcranial sonography.
Copyright © 2020 Zhang, Tao, Wang, Duan, Wang and Liu.
Figures


Similar articles
-
Clinical Features in Parkinson's Disease Patients with Hyperechogenicity in Substantia Nigra: A Cross-Sectional Study.Neuropsychiatr Dis Treat. 2022 Aug 2;18:1593-1601. doi: 10.2147/NDT.S374370. eCollection 2022. Neuropsychiatr Dis Treat. 2022. PMID: 35942277 Free PMC article.
-
Iron accumulation and microglia activation contribute to substantia nigra hyperechogenicity in the 6-OHDA-induced rat model of Parkinson's disease.Parkinsonism Relat Disord. 2017 Mar;36:76-82. doi: 10.1016/j.parkreldis.2017.01.003. Epub 2017 Jan 6. Parkinsonism Relat Disord. 2017. PMID: 28108264
-
Spatial variations and precise location of substantia nigra hyperechogenicity in Parkinson's disease using TCS-MR fusion imaging.NPJ Parkinsons Dis. 2025 Apr 16;11(1):78. doi: 10.1038/s41531-025-00910-7. NPJ Parkinsons Dis. 2025. PMID: 40240329 Free PMC article.
-
Application and progress of transcranial substantial ultrasound in Parkinson's disease.Front Neurol. 2022 Dec 1;13:1091895. doi: 10.3389/fneur.2022.1091895. eCollection 2022. Front Neurol. 2022. PMID: 36530621 Free PMC article. Review.
-
[Transcranial sonography of substantia nigra in patients with Parkinson's disease].Rinsho Shinkeigaku. 2013;53(11):981-2. doi: 10.5692/clinicalneurol.53.981. Rinsho Shinkeigaku. 2013. PMID: 24291852 Review. Japanese.
Cited by
-
Transcranial sonography with clinical and demographic characteristics to predict cognitive impairment in PD: a longitudinal study.BMC Neurol. 2023 Jan 13;23(1):15. doi: 10.1186/s12883-023-03057-1. BMC Neurol. 2023. PMID: 36639620 Free PMC article.
-
Clinical Features in Parkinson's Disease Patients with Hyperechogenicity in Substantia Nigra: A Cross-Sectional Study.Neuropsychiatr Dis Treat. 2022 Aug 2;18:1593-1601. doi: 10.2147/NDT.S374370. eCollection 2022. Neuropsychiatr Dis Treat. 2022. PMID: 35942277 Free PMC article.
-
Substantia nigra hyperechogenicity and brain ventricular size as biomarkers of early dementia with Lewy bodies.Alzheimers Res Ther. 2024 Oct 15;16(1):227. doi: 10.1186/s13195-024-01590-w. Alzheimers Res Ther. 2024. PMID: 39407323 Free PMC article.
-
Changes in the correlation between substantia nigra hyperechogenicity area and Parkinson's disease severity at different Hoehn and Yahr stages.Neurol Sci. 2024 Dec;45(12):5739-5747. doi: 10.1007/s10072-024-07697-0. Epub 2024 Aug 2. Neurol Sci. 2024. PMID: 39090356
-
Machine learning models for diagnosis and prognosis of Parkinson's disease using brain imaging: general overview, main challenges, and future directions.Front Aging Neurosci. 2023 Jul 19;15:1216163. doi: 10.3389/fnagi.2023.1216163. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37539346 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

