Mechanosensitivity of Human Oligodendrocytes
- PMID: 32848617
- PMCID: PMC7420028
- DOI: 10.3389/fncel.2020.00222
Mechanosensitivity of Human Oligodendrocytes
Abstract
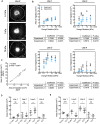
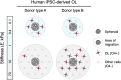
Oligodendrocytes produce and repair myelin, which is critical for the integrity and function of the central nervous system (CNS). Oligodendrocyte and oligodendrocyte progenitor cell (OPC) biology is modulated in vitro by mechanical cues within the magnitudes observed in vivo. In some cases, these cues are sufficient to accelerate or inhibit terminal differentiation of murine oligodendrocyte progenitors. However, our understanding of oligodendrocyte lineage mechanobiology has been restricted primarily to animal models to date, due to the inaccessibility and challenges of human oligodendrocyte cell culture. Here, we probe the mechanosensitivity of human oligodendrocyte lineage cells derived from human induced pluripotent stem cells. We target phenotypically distinct stages of the human oligodendrocyte lineage and quantify the effect of substratum stiffness on cell migration and differentiation, within the range documented in vivo. We find that human oligodendrocyte lineage cells exhibit mechanosensitive migration and differentiation. Further, we identify two patterns of human donor line-dependent mechanosensitive differentiation. Our findings illustrate the variation among human oligodendrocyte responses, otherwise not captured by animal models, that are important for translational research. Moreover, these findings highlight the importance of studying glia under conditions that better approximate in vivo mechanical cues. Despite significant progress in human oligodendrocyte derivation methodology, the extended duration, low yield, and low selectivity of human-induced pluripotent stem cell-derived oligodendrocyte protocols significantly limit the scale-up and implementation of these cells and protocols for in vivo and in vitro applications. We propose that mechanical modulation, in combination with traditional soluble and insoluble factors, provides a key avenue to address these challenges in cell production and in vitro analysis.
Keywords: glia; human models; in vitro; induced pluripotent stem cell; mechanobiology; mechanotransduction; oligodendrocyte.
Copyright © 2020 Espinosa-Hoyos, Burstein, Cha, Jain, Nijsure, Jagielska, Fossati and Van Vliet.
Figures


Similar articles
-
Progenitor-derived oligodendrocyte culture system from human fetal brain.J Vis Exp. 2012 Dec 20;(70):4274. doi: 10.3791/4274. J Vis Exp. 2012. PMID: 23288248 Free PMC article.
-
Differentiation of oligodendrocyte progenitor cells from dissociated monolayer and feeder-free cultured pluripotent stem cells.PLoS One. 2017 Feb 13;12(2):e0171947. doi: 10.1371/journal.pone.0171947. eCollection 2017. PLoS One. 2017. PMID: 28192470 Free PMC article.
-
Derivation of telencephalic oligodendrocyte progenitors from human pluripotent stem cells.Curr Protoc Stem Cell Biol. 2017 Nov 8;39:1H.10.1-1H.10.23. doi: 10.1002/cpsc.17. Curr Protoc Stem Cell Biol. 2017. PMID: 29081882 Free PMC article.
-
Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination.Biochim Biophys Acta. 2014 Sep;1843(9):1917-29. doi: 10.1016/j.bbamcr.2014.04.018. Epub 2014 Apr 21. Biochim Biophys Acta. 2014. PMID: 24768715 Review.
-
Modulation of Oligodendrocyte Differentiation by Mechanotransduction.Front Cell Neurosci. 2016 Nov 29;10:277. doi: 10.3389/fncel.2016.00277. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27965541 Free PMC article. Review.
Cited by
-
Piezo1-mediated spontaneous calcium transients in satellite glia impact dorsal root ganglia development.PLoS Biol. 2023 Sep 25;21(9):e3002319. doi: 10.1371/journal.pbio.3002319. eCollection 2023 Sep. PLoS Biol. 2023. PMID: 37747915 Free PMC article.
-
Novel Tools and Investigative Approaches for the Study of Oligodendrocyte Precursor Cells (NG2-Glia) in CNS Development and Disease.Front Cell Neurosci. 2021 Apr 29;15:673132. doi: 10.3389/fncel.2021.673132. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33994951 Free PMC article.
-
Tissue stiffness controls neuroblast migratory behavior and reprogramming during myelin repair.iScience. 2025 Jul 31;28(9):113255. doi: 10.1016/j.isci.2025.113255. eCollection 2025 Sep 19. iScience. 2025. PMID: 40837231 Free PMC article.
-
Artificial axons as a biomimetic 3D myelination platform for the discovery and validation of promyelinating compounds.Sci Rep. 2023 Nov 9;13(1):19529. doi: 10.1038/s41598-023-44675-6. Sci Rep. 2023. PMID: 37945646 Free PMC article.
-
Engineered cell culture microenvironments for mechanobiology studies of brain neural cells.Front Bioeng Biotechnol. 2022 Dec 14;10:1096054. doi: 10.3389/fbioe.2022.1096054. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36588937 Free PMC article. Review.
References
-
- Budday S., Ovaert T. C., Holzapfel G. A., Steinmann P., Kuhl E. (2019). Fifty Shades of Brain: A Review on the Mechanical Testing and Modeling of Brain Tissue. Netherlands: Springer.
Grants and funding
LinkOut - more resources
Full Text Sources

