The Mouse Levator Auris Longus Muscle: An Amenable Model System to Study the Role of Postsynaptic Proteins to the Maintenance and Regeneration of the Neuromuscular Synapse
- PMID: 32848618
- PMCID: PMC7405910
- DOI: 10.3389/fncel.2020.00225
The Mouse Levator Auris Longus Muscle: An Amenable Model System to Study the Role of Postsynaptic Proteins to the Maintenance and Regeneration of the Neuromuscular Synapse
Abstract
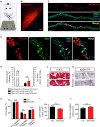
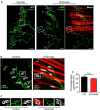
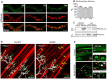
The neuromuscular junction (NMJ) is the peripheral synapse that controls the coordinated movement of many organisms. The NMJ is also an archetypical model to study synaptic morphology and function. As the NMJ is the primary target of neuromuscular diseases and traumatic injuries, the establishment of suitable models to study the contribution of specific postsynaptic muscle-derived proteins on NMJ maintenance and regeneration is a permanent need. Considering the unique experimental advantages of the levator auris longus (LAL) muscle, here we present a method allowing for efficient electroporation-mediated gene transfer and subsequent detailed studies of the morphology and function of the NMJ and muscle fibers. Also, we have standardized efficient facial nerve injury protocols to analyze LAL muscle NMJ degeneration and regeneration. Our results show that the expression of a control fluorescent protein does not alter either the muscle structural organization, the apposition of the pre- and post-synaptic domains, or the functional neurotransmission parameters of the LAL muscle NMJs; in turn, the overexpression of MuSK, a major regulator of postsynaptic assembly, induces the formation of ectopic acetylcholine receptor clusters. Our NMJ denervation experiments showed complete reinnervation of LAL muscle NMJs four weeks after facial nerve injury. Together, these experimental strategies in the LAL muscle constitute effective methods to combine protein expression with accurate analyses at the levels of structure, function, and regeneration of the NMJ.
Keywords: electroporation; neuromuscular junction; postsynaptic; presynaptic; regeneration; skeletal muscle.
Copyright © 2020 Ojeda, Bermedo-García, Pérez, Mella, Hanna, Herzberg, Tejero, López-Manzaneda, Tabares and Henríquez.
Figures



Similar articles
-
Combined In Vivo Electroporation and Short-Term Reinnervation of the Cranial Levator Auris Longus Skeletal Muscle.J Vis Exp. 2024 Nov 1;(213). doi: 10.3791/66706. J Vis Exp. 2024. PMID: 39555805
-
Functional regeneration of the murine neuromuscular synapse relies on long-lasting morphological adaptations.BMC Biol. 2022 Jul 8;20(1):158. doi: 10.1186/s12915-022-01358-4. BMC Biol. 2022. PMID: 35804361 Free PMC article.
-
The MuSK activator agrin has a separate role essential for postnatal maintenance of neuromuscular synapses.Proc Natl Acad Sci U S A. 2014 Nov 18;111(46):16556-61. doi: 10.1073/pnas.1408409111. Epub 2014 Nov 3. Proc Natl Acad Sci U S A. 2014. PMID: 25368159 Free PMC article.
-
Motor function recovery: deciphering a regenerative niche at the neuromuscular synapse.Biol Rev Camb Philos Soc. 2021 Apr;96(2):752-766. doi: 10.1111/brv.12675. Epub 2020 Dec 17. Biol Rev Camb Philos Soc. 2021. PMID: 33336525 Free PMC article. Review.
-
Maturation of a postsynaptic domain: Role of small Rho GTPases in organising nicotinic acetylcholine receptor aggregates at the vertebrate neuromuscular junction.J Anat. 2022 Nov;241(5):1148-1156. doi: 10.1111/joa.13526. Epub 2021 Aug 3. J Anat. 2022. PMID: 34342888 Free PMC article. Review.
Cited by
-
Selective Denervation of the Facial Dermato-Muscular Complex in the Rat: Experimental Model and Anatomical Basis.Front Neuroanat. 2021 Mar 22;15:650761. doi: 10.3389/fnana.2021.650761. eCollection 2021. Front Neuroanat. 2021. PMID: 33828465 Free PMC article.
-
Activation of TRPV1 Channels Inhibits the Release of Acetylcholine and Improves Muscle Contractility in Mice.Cell Mol Neurobiol. 2023 Nov;43(8):4157-4172. doi: 10.1007/s10571-023-01403-y. Epub 2023 Sep 9. Cell Mol Neurobiol. 2023. PMID: 37689594 Free PMC article.
-
Preliminary Observations on Skeletal Muscle Adaptation and Plasticity in Homer 2-/- Mice.Metabolites. 2021 Sep 19;11(9):642. doi: 10.3390/metabo11090642. Metabolites. 2021. PMID: 34564458 Free PMC article.
-
Impaired communication at the neuromotor axis during Degenerative Cervical Myelopathy.Front Cell Neurosci. 2024 Jan 10;17:1316432. doi: 10.3389/fncel.2023.1316432. eCollection 2023. Front Cell Neurosci. 2024. PMID: 38269114 Free PMC article.
-
Lithium causes differential effects on postsynaptic stability in normal and denervated neuromuscular synapses.Sci Rep. 2021 Aug 26;11(1):17285. doi: 10.1038/s41598-021-96708-7. Sci Rep. 2021. PMID: 34446751 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

