Purkinje Cell-Specific Knockout of Tyrosine Hydroxylase Impairs Cognitive Behaviors
- PMID: 32848620
- PMCID: PMC7403473
- DOI: 10.3389/fncel.2020.00228
Purkinje Cell-Specific Knockout of Tyrosine Hydroxylase Impairs Cognitive Behaviors
Abstract
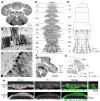
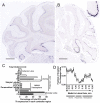
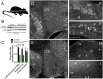
Tyrosine hydroxylase (Th) expression has previously been reported in Purkinje cells (PCs) of rodents and humans, but its role in the regulation of behavior is not understood. Catecholamines are well known for facilitating cognitive behaviors and are expressed in many regions of the brain. Here, we investigated a possible role in cognitive behaviors of PC catecholamines, by mapping and testing functional roles of Th positive PCs in mice. Comprehensive mapping analyses revealed a distinct population of Th expressing PCs primarily in the posterior and lateral regions of the cerebellum (comprising about 18% of all PCs). To identify the role of PC catecholamines, we selectively knocked out Th in PCs using a conditional knockout approach, by crossing a Purkinje cell-selective Cre recombinase line, Pcp2-Cre, with a floxed tyrosine hydroxylase mouse line (Thlox/lox) to produce Pcp2-Cre;Thlox/lox mice. This manipulation resulted in approximately 50% reduction of Th protein expression in the cerebellar cortex and lateral cerebellar nucleus, but no reduction of Th in the locus coeruleus, which is known to innervate the cerebellum in mice. Pcp2-Cre;Thlox/lox mice showed impairments in behavioral flexibility, response inhibition, social recognition memory, and associative fear learning relative to littermate controls, but no deficits in gross motor, sensory, instrumental learning, or sensorimotor gating functions. Catecholamines derived from specific populations of PCs appear to support cognitive functions, and their spatial distribution in the cerebellum suggests that they may underlie patterns of activation seen in human studies on the cerebellar role in cognitive function.
Keywords: catecholamine; cerebellum; cognition; dopamine; purkinje cell.
Copyright © 2020 Locke, Fujita, Hunker, Johanson, Darvas, du Lac, Zweifel and Carlson.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

