A Simple and Efficient Method for Visualizing Individual Cells in vivo by Cre-Mediated Single-Cell Labeling by Electroporation (CREMSCLE)
- PMID: 32848634
- PMCID: PMC7399061
- DOI: 10.3389/fncir.2020.00047
A Simple and Efficient Method for Visualizing Individual Cells in vivo by Cre-Mediated Single-Cell Labeling by Electroporation (CREMSCLE)
Abstract
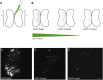
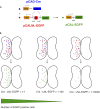
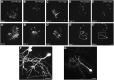
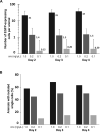
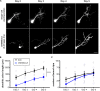
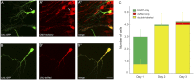
Efficient methods for visualizing cell morphology in the intact animal are of great benefit to the study of structural development in the nervous system. Quantitative analysis of the complex arborization patterns of brain cells informs cell-type classification, dissection of neuronal circuit wiring, and the elucidation of growth and plasticity mechanisms. Time-lapse single-cell morphological analysis requires labeling and imaging of single cells in situ without contamination from the ramified processes of other nearby cells. Here, using the Xenopus laevis optic tectum as a model system, we describe CRE-Mediated Single-Cell Labeling by Electroporation (CREMSCLE), a technique we developed based on bulk co-electroporation of Cre-dependent inducible expression vectors, together with very low concentrations of plasmid encoding Cre recombinase. This method offers efficient, sparse labeling in any brain area where bulk electroporation is possible. Unlike juxtacellular single-cell electroporation methods, CREMSCLE relies exclusively on the bulk electroporation technique, circumventing the need to precisely position a micropipette next to the target cell. Compared with viral transduction methods, it is fast and safe, generating high levels of expression within 24 h of introducing non-infectious plasmid DNA. In addition to increased efficiency of single-cell labeling, we confirm that CREMSCLE also allows for efficient co-expression of multiple gene products in the same cell. Furthermore, we demonstrate that this method is particularly well-suited for labeling immature neurons to follow their maturation over time. This approach therefore lends itself well to time-lapse morphological studies, particularly in the context of early neuronal development and under conditions that prevent more difficult visualized juxtacellular electroporation.
Keywords: Xenopus laevis; loxP; morphology; multiphoton; neuron; optic tectum; transfection.
Copyright © 2020 Schohl, Chorghay and Ruthazer.
Figures
Similar articles
-
Morpholino studies in Xenopus brain development.Methods Mol Biol. 2014;1082:155-71. doi: 10.1007/978-1-62703-655-9_11. Methods Mol Biol. 2014. PMID: 24048933
-
Single-cell electroporation of Xenopus tadpole tectal neurons.Cold Spring Harb Protoc. 2011 Sep 1;2011(9):pdb.prot065615. doi: 10.1101/pdb.prot065615. Cold Spring Harb Protoc. 2011. PMID: 21880812
-
Bulk electroporation of retinal ganglion cells in live Xenopus tadpoles.Cold Spring Harb Protoc. 2013 Aug 1;2013(8):771-5. doi: 10.1101/pdb.prot076471. Cold Spring Harb Protoc. 2013. PMID: 23906915
-
Labeling neurons in vivo for morphological and functional studies.Curr Opin Neurobiol. 2004 Oct;14(5):642-6. doi: 10.1016/j.conb.2004.08.007. Curr Opin Neurobiol. 2004. PMID: 15464899 Review.
-
Single-Cell Labeling Strategies to Dissect Neuronal Structures and Local Functions.Biology (Basel). 2023 Feb 16;12(2):321. doi: 10.3390/biology12020321. Biology (Basel). 2023. PMID: 36829594 Free PMC article. Review.
Cited by
-
Role of matrix metalloproteinase-9 in neurodevelopmental deficits and experience-dependent plasticity in Xenopus laevis.Elife. 2021 Jul 20;10:e62147. doi: 10.7554/eLife.62147. Elife. 2021. PMID: 34282726 Free PMC article.
-
In Utero Electroporation for Manipulation of Specific Neuronal Populations.Membranes (Basel). 2022 May 11;12(5):513. doi: 10.3390/membranes12050513. Membranes (Basel). 2022. PMID: 35629839 Free PMC article. Review.
-
The effects of the NMDAR co-agonist D-serine on the structure and function of optic tectal neurons in the developing visual system.Sci Rep. 2023 Aug 17;13(1):13383. doi: 10.1038/s41598-023-39951-4. Sci Rep. 2023. PMID: 37591903 Free PMC article.
-
Tissue-specific in vivo transformation of plasmid DNA in Neotropical tadpoles using electroporation.PLoS One. 2023 Aug 17;18(8):e0289361. doi: 10.1371/journal.pone.0289361. eCollection 2023. PLoS One. 2023. PMID: 37590232 Free PMC article.
-
Early Developmental Exposure to Fluoxetine and Citalopram Results in Different Neurodevelopmental Outcomes.Neuroscience. 2021 Jul 15;467:110-121. doi: 10.1016/j.neuroscience.2021.05.023. Epub 2021 May 25. Neuroscience. 2021. PMID: 34048796 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

