Activated Oxytocin Neurons in the PVN-DVC Pathway in Asthmatic Rats
- PMID: 32848637
- PMCID: PMC7412887
- DOI: 10.3389/fnana.2020.00047
Activated Oxytocin Neurons in the PVN-DVC Pathway in Asthmatic Rats
Abstract
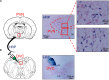
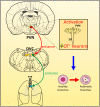
Asthma is a heterogeneous disease, and the central nervous system (CNS) also participates in the pathogenesis of asthma. We previously reported the amygdala might regulate asthmatic attacks via projecting to the paraventricular hypothalamic nucleus (PVN). The dorsal vagal complex (DVC) is a crucial region that modulates respiratory. This study aimed to observe the activity in both PVN and DVC and the connection between PVN and DVC in asthmatic rats. Immunohistochemistry was conducted to observe the changes in Fos and oxytocin (OT) expression. Retrograde tracing using wheat germ agglutinin-horseradish peroxidase (WGA-HRP) and double immunohistochemistry for OT and Fos was used to observe the HRP/OT/Fos positive neurons distribution in the PVN. The results showed that during an asthma attack, the Fos positive neurons increased in both PVN and DVC over time. The expression of OT positive neurons in PVN showed a similar trend in parallel to the c-Fos positive neurons in PVN. The HRP retrograde-labeled neurons were densely distributed in the medial and lateral subnucleus in the PVN. OT+/HRP+ and Fos+/OT+/HRP+ accounted for 18.14%, and 2.37% of HRP-labeled neurons, respectively. Our study showed PVN and DVC were activated and the expression of OT positive neurons in PVN were increased over time during an asthma attack. The existence of connection between PVN and DVC suggested the OT neurons in PVN might project to DVC which might be involved in the pathogenesis of asthma.
Keywords: asthma; dorsal vagal complex; oxytocin; paraventricular nucleus; pathway.
Copyright © 2020 Chen, Long, Xiao, Liu and Dong.
Figures




References
-
- Chen Z., Chen H., Chen F., Gu D., Sun L., Zhang W., et al. (2017). Vagotomy decreases the neuronal activities of medulla oblongata and alleviates neurogenic inflammation of airways induced by repeated intra-esophageal instillation of HCl in guinea pigs. Physiol. Res. 66 1021–1028. 10.33549/physiolres.933574 - DOI - PubMed
LinkOut - more resources
Full Text Sources

