Gut-Brain Axis in the Early Postnatal Years of Life: A Developmental Perspective
- PMID: 32848651
- PMCID: PMC7419604
- DOI: 10.3389/fnint.2020.00044
Gut-Brain Axis in the Early Postnatal Years of Life: A Developmental Perspective
Abstract
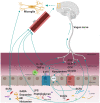
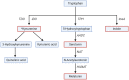
Emerging evidence suggests that alterations in the development of the gastrointestinal (GI) tract during the early postnatal period can influence brain development and vice-versa. It is increasingly recognized that communication between the GI tract and brain is mainly driven by neural, endocrine, immune, and metabolic mediators, collectively called the gut-brain axis (GBA). Changes in the GBA mediators occur in response to the developmental changes in the body during this period. This review provides an overview of major developmental events in the GI tract and brain in the early postnatal period and their parallel developmental trajectories under physiological conditions. Current knowledge of GBA mediators in context to brain function and behavioral outcomes and their synthesis and metabolism (site, timing, etc.) is discussed. This review also presents hypotheses on the role of the GBA mediators in response to the parallel development of the GI tract and brain in infants.
Keywords: brain; cognition; gastrointestinal tract; gut-brain axis; metabolites; microbiota; postnatal development.
Copyright © 2020 Jena, Montoya, Mullaney, Dilger, Young, McNabb and Roy.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

