The Neuroscience of Spatial Navigation and the Relationship to Artificial Intelligence
- PMID: 32848684
- PMCID: PMC7399088
- DOI: 10.3389/fncom.2020.00063
The Neuroscience of Spatial Navigation and the Relationship to Artificial Intelligence
Abstract
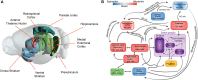
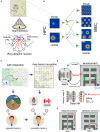
Recent advances in artificial intelligence (AI) and neuroscience are impressive. In AI, this includes the development of computer programs that can beat a grandmaster at GO or outperform human radiologists at cancer detection. A great deal of these technological developments are directly related to progress in artificial neural networks-initially inspired by our knowledge about how the brain carries out computation. In parallel, neuroscience has also experienced significant advances in understanding the brain. For example, in the field of spatial navigation, knowledge about the mechanisms and brain regions involved in neural computations of cognitive maps-an internal representation of space-recently received the Nobel Prize in medicine. Much of the recent progress in neuroscience has partly been due to the development of technology used to record from very large populations of neurons in multiple regions of the brain with exquisite temporal and spatial resolution in behaving animals. With the advent of the vast quantities of data that these techniques allow us to collect there has been an increased interest in the intersection between AI and neuroscience, many of these intersections involve using AI as a novel tool to explore and analyze these large data sets. However, given the common initial motivation point-to understand the brain-these disciplines could be more strongly linked. Currently much of this potential synergy is not being realized. We propose that spatial navigation is an excellent area in which these two disciplines can converge to help advance what we know about the brain. In this review, we first summarize progress in the neuroscience of spatial navigation and reinforcement learning. We then turn our attention to discuss how spatial navigation has been modeled using descriptive, mechanistic, and normative approaches and the use of AI in such models. Next, we discuss how AI can advance neuroscience, how neuroscience can advance AI, and the limitations of these approaches. We finally conclude by highlighting promising lines of research in which spatial navigation can be the point of intersection between neuroscience and AI and how this can contribute to the advancement of the understanding of intelligent behavior.
Keywords: artificial intelligence; deep learning; learning; memory; neuroscience; reinforcement learning; spatial navigation.
Copyright © 2020 Bermudez-Contreras, Clark and Wilber.
Figures

References
-
- Alemi A. A., Fischer I., Dillon J. V., Murphy K. (2019). Deep variational information bottleneck, in 5th International Conference on Learning Representations ICLR 2017e Track Proc (Toulon: ), 1–19.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

