Enteral Lactoferrin Supplementation for Preventing Sepsis and Necrotizing Enterocolitis in Preterm Infants: A Meta‑Analysis With Trial Sequential Analysis of Randomized Controlled Trials
- PMID: 32848789
- PMCID: PMC7426497
- DOI: 10.3389/fphar.2020.01186
Enteral Lactoferrin Supplementation for Preventing Sepsis and Necrotizing Enterocolitis in Preterm Infants: A Meta‑Analysis With Trial Sequential Analysis of Randomized Controlled Trials
Abstract
Background: Several clinical trials investigated the effects of enteral lactoferrin supplementation on the prevention of sepsis and necrotizing enterocolitis (NEC) in preterm infants, but the efficacy and safety remain disputed. Therefore, we systematically evaluated the effect of enteral lactoferrin supplementation in preterm infants through a meta‑analysis with trial sequential analysis (TSA).
Methods: We searched six databases to identify randomized controlled trials (RCTs) that evaluated the effects of lactoferrin supplementation compared with placebo or no intervention in preterm infants. RevMan version 5.3 software was used to estimate pooled relative risks (RRs) with the random-effects model. TSA, subgroup analyses, and meta-regression analyses were also performed.
Results: Nine RCTs with 3515 samples were included. With low to moderate quality of evidence, compared with placebo, enteral lactoferrin supplementation did not significantly decrease the incidences of late-onset sepsis (RR = 0.63, 95% CI: 0.38 to 1.02, P = 0.06), NEC stage II or III (RR = 0.68, 95% CI: 0.30 to 1.52, P = 0.35), all-cause mortality (RR = 0.89, 95% CI: 0.51 to 1.57, P = 0.69), bronchopulmonary dysplasia (RR = 1.01, 95% CI: 0.90 to 1.13, P = 0.92), retinopathy of prematurity (RR = 0.80, 95% CI: 0.49 to 1.32, P = 0.38), invasive fungal infection (RR = 0.27, 95% CI: 0.02 to 3.94, P = 0.34), intraventricular hemorrhage (RR = 1.40, 95% CI: 0.39 to 5.08, P = 0.61), and urinary tract infection (RR = 0.35, 95% CI: 0.11 to 1.06, P = 0.06). Subgroup analysis revealed that lactoferrin significantly reduced the incidence of sepsis in infants with a birth weight below 1500 g (RR = 0.43, 95% CI: 0.22 to 0.84, P = 0.01). TSAs of the primary outcomes showed that the evidence is insufficient and further data is required.
Conclusions: Limited evidence suggested that enteral lactoferrin supplementation was associated with a reduction of late-onset sepsis in infants with a birth weight below 1500g, however, did not decrease the incidence of NEC stage II or III, all-cause mortality, and other adverse events in preterm infants. The present evidence was insufficient to inform clinical practice.
Keywords: lactoferrin; meta‑analysis; mortality; necrotizing enterocolitis; preterm infant; sepsis; trial sequential analysis.
Copyright © 2020 Gao, Hou, Lu, Wang, Pan, Wang, Tian and Ge.
Figures


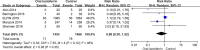


References
-
- Bangalore S., Kumar S., Kjeldsen S. E., Makani H., Grossman E., Wetterslev J., et al. (2011). Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. 12, 65–82. 10.1016/S1470-2045(10)70260-6 - DOI - PubMed
-
- Bassler D., Stoll B. J., Schmidt B., Asztalos E. V., Roberts R. S., Robertson C. M., et al. (2009). Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: added role of neonatal infection. Pediatrics 123, 313–318. 10.1542/peds.2008-0377 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

