Can Cephalopods Vomit? Hypothesis Based on a Review of Circumstantial Evidence and Preliminary Experimental Observations
- PMID: 32848811
- PMCID: PMC7396502
- DOI: 10.3389/fphys.2020.00765
Can Cephalopods Vomit? Hypothesis Based on a Review of Circumstantial Evidence and Preliminary Experimental Observations
Abstract
In representative species of all vertebrate classes, the oral ejection of upper digestive tract contents by vomiting or regurgitation is used to void food contaminated with toxins or containing indigestible material not voidable in the feces. Vomiting or regurgitation has been reported in a number of invertebrate marine species (Exaiptasia diaphana, Cancer productus, and Pleurobranchaea californica), prompting consideration of whether cephalopods have this capability. This "hypothesis and theory" paper reviews four lines of supporting evidence: (1) the mollusk P. californica sharing some digestive tract morphological and innervation similarities with Octopus vulgaris is able to vomit or regurgitate with the mechanisms well characterized, providing an example of motor program switching; (2) a rationale for vomiting or regurgitation in cephalopods based upon the potential requirement to void indigestible material, which may cause damage and ejection of toxin contaminated food; (3) anecdotal reports (including from the literature) of vomiting- or regurgitation-like behavior in several species of cephalopod (Sepia officinalis, Sepioteuthis sepioidea, O. vulgaris, and Enteroctopus dofleini); and (4) anatomical and physiological studies indicating that ejection of gastric/crop contents via the buccal cavity is a theoretical possibility by retroperistalsis in the upper digestive tract (esophagus, crop, and stomach). We have not identified any publications refuting our hypothesis, so a balanced review is not possible. Overall, the evidence presented is circumstantial, so experiments adapting current methodology (e.g., research community survey, in vitro studies of motility, and analysis of indigestible gut contents and feces) are described to obtain additional evidence to either support or refute our hypothesis. We recognize the possibility that further research may not support the hypothesis; therefore, we consider how cephalopods may protect themselves against ingestion of toxic food by external chemodetection prior to ingestion and digestive gland detoxification post-ingestion. Reviewing the evidence for the hypothesis has identified a number of gaps in knowledge of the anatomy (e.g., the presence of sphincters) and physiology (e.g., the fate of indigestible food residues, pH of digestive secretions, sensory innervation, and digestive gland detoxification mechanisms) of the digestive tract as well as a paucity of recent studies on the role of epithelial chemoreceptors in prey identification and food intake.
Keywords: Octopus vulgaris; Sepia officinalis; digestive tract; motility; nutrition; regurgitation; vomiting; welfare.
Copyright © 2020 Sykes, Almansa, Ponte, Cooke and Andrews.
Figures

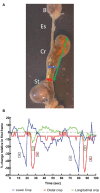
Similar articles
-
Methodological considerations in studying digestive system physiology in octopus: limitations, lacunae and lessons learnt.Front Physiol. 2022 Sep 9;13:928013. doi: 10.3389/fphys.2022.928013. eCollection 2022. Front Physiol. 2022. PMID: 36160859 Free PMC article. Review.
-
The Digestive Tract of Cephalopods: a Neglected Topic of Relevance to Animal Welfare in the Laboratory and Aquaculture.Front Physiol. 2017 Jul 17;8:492. doi: 10.3389/fphys.2017.00492. eCollection 2017. Front Physiol. 2017. PMID: 28769814 Free PMC article. Review.
-
Prey Capture, Ingestion, and Digestion Dynamics of Octopus vulgaris Paralarvae Fed Live Zooplankton.Front Physiol. 2017 Aug 17;8:573. doi: 10.3389/fphys.2017.00573. eCollection 2017. Front Physiol. 2017. PMID: 28860996 Free PMC article.
-
B-esterases characterisation in the digestive tract of the common octopus and the European cuttlefish and their in vitro responses to contaminants of environmental concern.Environ Res. 2022 Jul;210:112961. doi: 10.1016/j.envres.2022.112961. Epub 2022 Feb 16. Environ Res. 2022. PMID: 35181305
-
The Digestive Tract of Cephalopods: Toward Non-invasive In vivo Monitoring of Its Physiology.Front Physiol. 2017 Jun 19;8:403. doi: 10.3389/fphys.2017.00403. eCollection 2017. Front Physiol. 2017. PMID: 28674501 Free PMC article.
Cited by
-
Methodological considerations in studying digestive system physiology in octopus: limitations, lacunae and lessons learnt.Front Physiol. 2022 Sep 9;13:928013. doi: 10.3389/fphys.2022.928013. eCollection 2022. Front Physiol. 2022. PMID: 36160859 Free PMC article. Review.
References
-
- Alexandrowicz J. S. (1928). Notes sur l’innervation du tube digestif des cephalopods. Arch. Zool. Exp. Gen. 67, 69–90.
-
- Altman J. S., Nixon M. (1970). Use of beaks and radula by Octopus vulgaris in feeding. J. Zool. 161, 25–38. 10.1111/j.1469-7998.1970.tb02167.x - DOI
-
- Anadon R. (2019). “Functional histology: the tissues of common coleoid cephalopods” in Handbook of pathogens and diseases in cephalopods. eds. Gestal C., Pascual S., Guerra A., Fiorito G., Vieites J. M. (Switzerland AG: Springer Open, Springer Nature; ), 230.
LinkOut - more resources
Full Text Sources

