Non-invasive Auricular Vagus Nerve Stimulation as a Potential Treatment for Covid19-Originated Acute Respiratory Distress Syndrome
- PMID: 32848845
- PMCID: PMC7399203
- DOI: 10.3389/fphys.2020.00890
Non-invasive Auricular Vagus Nerve Stimulation as a Potential Treatment for Covid19-Originated Acute Respiratory Distress Syndrome
Abstract
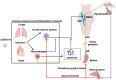
Background: Covid-19 is an infectious disease caused by an invasion of the alveolar epithelial cells by coronavirus 19. The most severe outcome of the disease is the Acute Respiratory Distress Syndrome (ARDS) combined with hypoxemia and cardiovascular damage. ARDS and co-morbidities are associated with inflammatory cytokine storms, sympathetic hyperactivity, and respiratory dysfunction. Hypothesis: In the present paper, we present and justify a novel potential treatment for Covid19-originated ARDS and associated co-morbidities, based on the non-invasive stimulation of the auricular branch of the vagus nerve. Methods: Auricular vagus nerve stimulation activates the parasympathetic system including anti-inflammatory pathways (the cholinergic anti-inflammatory pathway and the hypothalamic pituitary adrenal axis) while regulating the abnormal sympatho-vagal balance and improving respiratory control. Results: Along the paper (1) we expose the role of the parasympathetic system and the vagus nerve in the control of inflammatory processes (2) we formulate our physiological and methodological hypotheses (3) we provide a large body of clinical and preclinical data that support the favorable effects of auricular vagus nerve stimulation in inflammation, sympatho-vagal balance as well as in respiratory and cardiac ailments, and (4) we list the (few) possible collateral effects of the treatment. Finally, we discuss auricular vagus nerve stimulation protective potential, especially in the elderly and co-morbid population with already reduced parasympathetic response. Conclusions: Auricular vagus nerve stimulation is a safe clinical procedure and it could be either an effective treatment for ARDS originated by Covid-19 and similar viruses or a supplementary treatment to actual ARDS therapeutic approaches.
Keywords: cholinergic anti-inflammatory pathway; hypothalamic pituitary adrenal axis; lung inflammation; parasympathetic system; sympatho-vagal balance.
Copyright © 2020 Kaniusas, Szeles, Kampusch, Alfageme-Lopez, Yucuma-Conde, Li, Mayol, Neumayer, Papa and Panetsos.
Figures
References
-
- Afanasiev S. A., Pavliukova E. N., Kuzmichkina M. A., Rebrova T. Y., Anfinogenova Y., Likhomanov K. S., et al. (2016). Nonpharmacological correction of hypersympatheticotonia in patients with chronic coronary insufficiency and severe left ventricular dysfunction. Ann. Noninvasive Electrocardiol. 21, 548–556. 10.1111/anec.12349 - DOI - PMC - PubMed
-
- Annoni E. M., Xie X., Lee S. W., Libbus I., KenKnight B. H., Osborn J. W., et al. (2015). Intermittent electrical stimulation of the right cervical vagus nerve in salt-sensitive hypertensive rats: effects on blood pressure, arrhythmias, and ventricular electrophysiology. Physiol. Rep. 3:e12476. 10.14814/phy2.12476 - DOI - PMC - PubMed
-
- Antonino D., Teixeira A. L., Maia-Lopes P. M., Souza M. C., Sabino-Carvalho J. L., Murray A. R., et al. (2017). Non-invasive vagus nerve stimulation acutely improves spontaneous cardiac baroreflex sensitivity in healthy young men: a randomized placebo-controlled trial. Brain Stimul. 10, 875–881. 10.1016/j.brs.2017.05.006 - DOI - PubMed
-
- Babygirija R., Sood M., Kannampalli P., Sengupta J. N., Miranda A. (2017). Percutaneous electrical nerve field stimulation modulates central pain pathways and attenuates post-inflammatory visceral and somatic hyperalgesia in rats. Neuroscience 356, 11–21. 10.1016/j.neuroscience.2017.05.012 - DOI - PubMed
LinkOut - more resources
Full Text Sources

