Interleukin (IL)-6: A Friend or Foe of Pregnancy and Parturition? Evidence From Functional Studies in Fetal Membrane Cells
- PMID: 32848846
- PMCID: PMC7397758
- DOI: 10.3389/fphys.2020.00891
Interleukin (IL)-6: A Friend or Foe of Pregnancy and Parturition? Evidence From Functional Studies in Fetal Membrane Cells
Abstract
Objective: Protection of the fetus within the amniotic sac is primarily attained by remodeling fetal membrane (amniochorion) cells through cyclic epithelial to mesenchymal and mesenchymal to epithelial (EMT and MET) transitions. Endocrine and paracrine factors regulate EMT and MET during pregnancy. At term, increased oxidative stress forces a terminal state of EMT and inflammation, predisposing to membrane weakening and rupture. IL-6 is a constitutively expressed cytokine during gestation, but it is elevated in term and preterm births. Therefore, we tested the hypothesis that IL-6 can determine the fate of amnion membrane cells and that pathologic levels of IL-6 can cause a terminal state of EMT and inflammation, leading to adverse pregnancy outcomes.
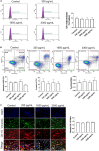
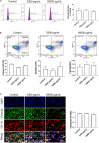
Methods: Primary amnion epithelial cells (AECs) were treated with recombinant IL-6 (330, 1,650, 3,330, and 16,000 pg/ml) for 48 h (N = 5). IL-6-induced cell senescence (aging), cell death (apoptosis and necrosis), and cell cycle changes were studied using flow cytometry. Cellular transitions were determined by immunocytochemistry and western blot analysis, while IL-6 signaling (activation of signaling kinases) was measured by immunoassay. Inflammatory marker matrix metalloproteinase (MMP9) and granulocyte-macrophage colony-stimulating factor (GM-CSF) concentrations were measured using a Fluorokine E assay and ELISA, respectively. Amniotic membranes collected on gestational day (D) 12 and D18 from IL-6 knockout (KO) and control C57BL/6 mice (N = 3 each) were used to determine the impact of IL-6 on cell transitions. Fold changes were measured based on the mean of each group.
Results: IL-6 treatment of AECs at physiologic or pathologic doses increased JNK and p38MAPK activation; however, the activation of signals did not cause changes in AEC cell cycle, cellular senescence, apoptosis, necrosis, cellular transitions, or inflammation (MMP9 and GM-CSF) compared to control. EMT markers were higher on D18 compared to D12 regardless of IL-6 status in the mouse amniotic sac.
Conclusion: Physiologic and pathologic concentrations of IL-6 did not cause amnion cell aging, cell death, cellular transitions, or inflammation. IL-6 may function to maintain cellular homeostasis throughout gestation in fetal membrane cells. Although IL-6 is a good biomarker for adverse pregnancies, it is not an indicator of an underlying pathological mechanism in membrane cells.
Keywords: EMT; amniotic epithelial cells (AECs); cytokines; fetal membranes; inflammation.
Copyright © 2020 Omere, Richardson, Saade, Bonney, Kechichian and Menon.
Figures



References
-
- Blencowe H., Cousens S., Oestergaard M. Z., Chou D., Moller A. B., Narwal R., et al. (2012). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379 2162–2172. 10.1016/s0140-6736(12)60820-4 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

