Chemerin Added to Endothelin-1 Promotes Rat Pulmonary Artery Smooth Muscle Cell Proliferation and Migration
- PMID: 32848866
- PMCID: PMC7406802
- DOI: 10.3389/fphys.2020.00926
Chemerin Added to Endothelin-1 Promotes Rat Pulmonary Artery Smooth Muscle Cell Proliferation and Migration
Abstract
Background: While chemerin has been shown to increase proliferation and migration of systemic vascular smooth muscle cells (SMCs) contributing therefore to the development of hypertension, this remains to be clarified for the pulmonary circulation.
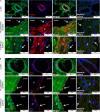
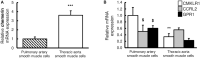
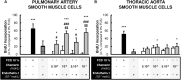
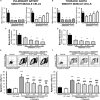
Methods: Expression of chemerin and its three receptors (CMKRL1, CCRL2, GPR1) was examined by immunohistochemistry and RTq-PCR in lungs, pulmonary artery, and thoracic aorta from Wistar rats. Primary cultured rat pulmonary artery and thoracic aorta SMCs treated with recombinant chemerin (tested from 5.10-9 to 10-7 mol/L) were assessed for proliferation and migration (both with 10-7 mol/L endothelin-1), as well as for staurosporine-induced apoptosis.
Results: In pulmonary artery and thoracic aorta, CMKLR1 expression was detected in both endothelial cells and SMCs. In primary cultured pulmonary artery SMCs, chemerin and its three receptors were expressed, and CMKLR1 expression was higher than those of CCRL2 and GPR1. Chemerin added to endothelin-1 increased pulmonary artery SMC proliferation, while chemerin or endothelin-1 alone did not. This effect was less pronounced in thoracic aorta SMCs. Chemerin induced pulmonary artery and thoracic aorta SMC migration, which was exacerbated by endothelin-1 and more pronounced in thoracic aorta SMCs. Chemerin concentration-dependently reduced staurosporine-induced apoptosis in both pulmonary artery and thoracic aorta SMCs. In pulmonary artery SMCs, endothelin-1 treatment increased the expression of CMKLR1, CCRL2, and GPR1, while these expressions were not altered in thoracic aorta SMCs.
Conclusion: Chemerin/CMKRL1 signaling, in conjunction with a key mediator in the pathogenesis of pulmonary hypertensive diseases, endothelin-1, stimulated proliferation and migration, and increased resistance to apoptosis in rat primary cultured pulmonary artery SMCs. Our results suggest that this signaling could play a role in pulmonary artery remodeling observed in pulmonary hypertension.
Keywords: CMKRL1; apoptosis; chemerin; endothelin-1; migration; proliferation; pulmonary artery; smooth muscle cells.
Copyright © 2020 Hanthazi, Jespers, Vegh, Dubois, Hubesch, Springael, Dewachter and Mc Entee.
Figures

Similar articles
-
Chemerin stimulates aortic smooth muscle cell proliferation and migration via activation of autophagy in VSMCs of metabolic hypertension rats.Am J Transl Res. 2019 Mar 15;11(3):1327-1342. eCollection 2019. Am J Transl Res. 2019. PMID: 30972165 Free PMC article.
-
Endothelin-1 induces pulmonary but not aortic smooth muscle cell migration by activating ERK1/2 MAP kinase.Can J Physiol Pharmacol. 2010 Aug;88(8):830-9. doi: 10.1139/Y10-059. Can J Physiol Pharmacol. 2010. PMID: 20725141
-
Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure.Am J Physiol Heart Circ Physiol. 2015 Sep;309(5):H1017-28. doi: 10.1152/ajpheart.00820.2014. Epub 2015 Aug 7. Am J Physiol Heart Circ Physiol. 2015. PMID: 26254337
-
Structural Basis for Chemerin Recognition and Signaling Through Its Receptors.Biomedicines. 2024 Oct 28;12(11):2470. doi: 10.3390/biomedicines12112470. Biomedicines. 2024. PMID: 39595036 Free PMC article. Review.
-
Chemerin: A Functional Adipokine in Reproductive Health and Diseases.Biomedicines. 2022 Aug 7;10(8):1910. doi: 10.3390/biomedicines10081910. Biomedicines. 2022. PMID: 36009457 Free PMC article. Review.
Cited by
-
Superficial Parasternal Intercostal Plane Block and Full Sternotomy; A Randomized Trial.Eur J Cardiothorac Surg. 2025 Jul 1;67(7):ezaf226. doi: 10.1093/ejcts/ezaf226. Eur J Cardiothorac Surg. 2025. PMID: 40627369 Free PMC article. Clinical Trial.
-
Adipokines in pulmonary hypertension: angels or demons?Heliyon. 2023 Nov 17;9(11):e22482. doi: 10.1016/j.heliyon.2023.e22482. eCollection 2023 Nov. Heliyon. 2023. PMID: 38074873 Free PMC article. Review.
-
Role of Chemerin/ChemR23 axis as an emerging therapeutic perspective on obesity-related vascular dysfunction.J Transl Med. 2022 Mar 22;20(1):141. doi: 10.1186/s12967-021-03220-7. J Transl Med. 2022. PMID: 35317838 Free PMC article. Review.
-
Biomarkers of haemodynamic severity of systemic sclerosis-associated pulmonary arterial hypertension by serum proteome analysis.Ann Rheum Dis. 2023 Mar;82(3):365-373. doi: 10.1136/ard-2022-223237. Epub 2022 Dec 5. Ann Rheum Dis. 2023. PMID: 36600187 Free PMC article.
-
Vascular effects of perivascular adipose tissue-derived chemerin in obesity-associated cardiovascular disease.Cardiovasc Diabetol. 2025 Jun 13;24(1):249. doi: 10.1186/s12933-025-02814-5. Cardiovasc Diabetol. 2025. PMID: 40514684 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

