Structural and Functional Remodeling of the Brain Vasculature Following Stroke
- PMID: 32848875
- PMCID: PMC7433746
- DOI: 10.3389/fphys.2020.00948
Structural and Functional Remodeling of the Brain Vasculature Following Stroke
Abstract
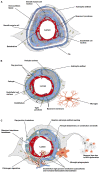
Maintenance of cerebral blood vessel integrity and regulation of cerebral blood flow ensure proper brain function. The adult human brain represents only a small portion of the body mass, yet about a quarter of the cardiac output is dedicated to energy consumption by brain cells at rest. Due to a low capacity to store energy, brain health is heavily reliant on a steady supply of oxygen and nutrients from the bloodstream, and is thus particularly vulnerable to stroke. Stroke is a leading cause of disability and mortality worldwide. By transiently or permanently limiting tissue perfusion, stroke alters vascular integrity and function, compromising brain homeostasis and leading to widespread consequences from early-onset motor deficits to long-term cognitive decline. While numerous lines of investigation have been undertaken to develop new pharmacological therapies for stroke, only few advances have been made and most clinical trials have failed. Overall, our understanding of the acute and chronic vascular responses to stroke is insufficient, yet a better comprehension of cerebrovascular remodeling following stroke is an essential prerequisite for developing novel therapeutic options. In this review, we present a comprehensive update on post-stroke cerebrovascular remodeling, an important and growing field in neuroscience, by discussing cellular and molecular mechanisms involved, sex differences, limitations of preclinical research design and future directions.
Keywords: angiogenesis; blood–brain barrier; cerebrovascular; neurovascular unit; stroke; vascular remodeling.
Copyright © 2020 Freitas-Andrade, Raman-Nair and Lacoste.
Figures
References
-
- Albargothy N. J., Johnston D. A., MacGregor-Sharp M., Weller R. O., Verma A., Hawkes C. A., et al. (2018). Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 136 139–152. 10.1007/s00401-018-1862-7 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

