The Enigma of Endothelium in COVID-19
- PMID: 32848893
- PMCID: PMC7417426
- DOI: 10.3389/fphys.2020.00989
The Enigma of Endothelium in COVID-19
Abstract
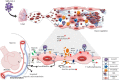
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome-related coronavirus-2 (SARS-CoV-2) has affected millions of people globally. Clinically, it presents with mild flu-like symptoms in most cases but can cause respiratory failure in high risk population. With the aim of unearthing newer treatments, scientists all over the globe are striving hard to comprehend the underlying mechanisms of COVID-19. Several studies till date have indicated a dysregulated host immune response as the major cause of COVID-19 induced mortality. In this Perspective, we propose a key role of endothelium, particularly pulmonary endothelium in the pathogenesis of COVID-19. We draw parallels and divergences between COVID-19-induced respiratory distress and bacterial sepsis-induced lung injury and recommend the road ahead with respect to identification of endothelium-based biomarkers and plausible treatments for COVID-19.
Keywords: COVID-19; coagulation; endothelial dysfunction; inflammation; pathogenesis and diagnosis; vascular biology; viral sepsis.
Copyright © 2020 Kaur, Tripathi and Yadav.
Figures
References
-
- Aird W. C. (2007). Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circul. Res. 100 174–190. 10.1161/01.res.0000255690.03436.ae - DOI - PubMed
-
- Colmenero I., Santonja C., Alonso-Riaño M., Noguera-Morel L., Hernández-Martín A., Andina D., et al. (2020). SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultraestructural study of 7 paediatric cases. Br. J. Dermatol.. 10.1111/bjd.19327 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

