Raising Placebo Efficacy in Antidepressant Trials Across Decades Explained by Small-Study Effects: A Meta-Reanalysis
- PMID: 32848900
- PMCID: PMC7399231
- DOI: 10.3389/fpsyt.2020.00633
Raising Placebo Efficacy in Antidepressant Trials Across Decades Explained by Small-Study Effects: A Meta-Reanalysis
Abstract
Background: Recent meta-analyses reported placebo response rate in antidepressant trials to be stable since the 1970s. These meta-analyses however were limited in considering only linear time trends, assessed trial-level covariates based on single-model hypothesis testing only, and did not adjust for small-study effects (SSE), a well-known but not yet formally assessed bias in antidepressant trials.
Methods: This secondary meta-analysis extends previous work by modeling nonlinear time trends, assessing the relative importance of trial-level covariates using a multimodel approach, and rigorously adjusting for SSE. Outcomes were placebo efficacy (continuous), based on the Hamilton Depression Scale, and placebo response rate.
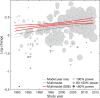
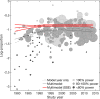
Results: Results suggested that any nonlinear time trends in both placebo efficacy (continuous) and response rate were best explained by SSE. Adjusting for SSE revealed a significant gradual increase in placebo efficacy (continuous) from 1979 to 2014. A similar observation was made for placebo response rate, but did not reach significance due higher susceptibility to SSE. By contrast, trial-level covariates alone were found to be insufficient in explaining time trends.
Conclusion: The present findings contribute to the ongoing debate on antidepressant placebo outcomes and highlight the need to adjust for bias introduced by SSE. The results are of clinical relevance because SSE may affect the evaluation of success or failure in antidepressant trials.
Keywords: antidepressants; meta-analysis; placebo; small-study effects; time trend.
Copyright © 2020 Holper.
Figures
Similar articles
-
Adjuvant therapy with antidepressants for the management of inflammatory bowel disease.Cochrane Database Syst Rev. 2019 Apr 12;4(4):CD012680. doi: 10.1002/14651858.CD012680.pub2. Cochrane Database Syst Rev. 2019. PMID: 30977111 Free PMC article.
-
Efficacy of new-generation antidepressants assessed with the Montgomery-Asberg Depression Rating Scale, the gold standard clinician rating scale: A meta-analysis of randomised placebo-controlled trials.PLoS One. 2020 Feb 26;15(2):e0229381. doi: 10.1371/journal.pone.0229381. eCollection 2020. PLoS One. 2020. PMID: 32101579 Free PMC article.
-
Has the rising placebo response impacted antidepressant clinical trial outcome? Data from the US Food and Drug Administration 1987-2013.World Psychiatry. 2017 Jun;16(2):181-192. doi: 10.1002/wps.20421. World Psychiatry. 2017. PMID: 28498591 Free PMC article.
-
Meta-analysis of placebo group dropout in adult antidepressant trials.Prog Neuropsychopharmacol Biol Psychiatry. 2020 Mar 2;98:109777. doi: 10.1016/j.pnpbp.2019.109777. Epub 2019 Nov 5. Prog Neuropsychopharmacol Biol Psychiatry. 2020. PMID: 31697973
-
Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: a meta-analysis.J Clin Psychiatry. 2012 Oct;73(10):1300-6. doi: 10.4088/JCP.11r07485. J Clin Psychiatry. 2012. PMID: 23140647 Review.
Cited by
-
Predicting Antidepressant Effects of Ketamine: the Role of the Pregenual Anterior Cingulate Cortex as a Multimodal Neuroimaging Biomarker.Int J Neuropsychopharmacol. 2022 Dec 12;25(12):1003-1013. doi: 10.1093/ijnp/pyac049. Int J Neuropsychopharmacol. 2022. PMID: 35948274 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

