Relapsing Demyelinating Syndromes in Children: A Practical Review of Neuroradiological Mimics
- PMID: 32849169
- PMCID: PMC7417677
- DOI: 10.3389/fneur.2020.00627
Relapsing Demyelinating Syndromes in Children: A Practical Review of Neuroradiological Mimics
Abstract
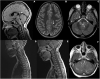
Relapsing demyelinating syndromes (RDS) in children encompass a diverse spectrum of entities including multiple sclerosis (MS) acute disseminated encephalomyelitis (ADEM), aquaporin-4 antibody associated neuromyelitis optica spectrum disorder (AQP4-NMOSD) and myelin oligodendrocyte glycoprotein antibody disease (MOG-AD). In addition to these, there are "antibody-negative" demyelinating syndromes which are yet to be fully characterized and defined. The paucity of specific biomarkers and overlap in clinical presentations makes the distinction between these disease entities difficult at initial presentation and, as such, there is a heavy reliance on magnetic resonance imaging (MRI) findings to satisfy the criteria for treatment initiation and optimization. Misdiagnosis is not uncommon and is usually related to the inaccurate application of criteria or failure to identify potential clinical and radiological mimics. It is also notable that there are instances where AQP4 and MOG antibody testing may be falsely negative during initial clinical episodes, further complicating the issue. This article illustrates the typical clinico-radiological phenotypes associated with the known pediatric RDS at presentation and describes the neuroimaging mimics of these using a pattern-based approach in the brain, optic nerves, and spinal cord. Practical guidance on key distinguishing features in the form of clinical and radiological red flags are incorporated. A subsection on clinical mimics with characteristic imaging patterns that assist in establishing alternative diagnoses is also included.
Keywords: ADEM; AQP4; MOG; MS; demyelimating disease; mimics; multiple scleorsis; pediatric.
Copyright © 2020 Chhabda, Malik, Reddy, Muthusamy, Mirsky, Sudhakar and Mankad.
Figures
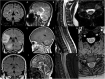
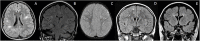
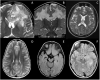
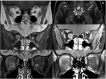
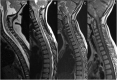
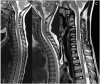
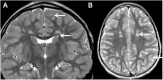
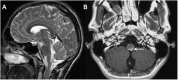
References
-
- Fadda G, Brown RA, Longoni G, Castro DA, O'Mahony J, Verhey LH, et al. . MRI and laboratory features and the performance of international criteria in the diagnosis of multiple sclerosis in children and adolescents: a prospective cohort study. Lancet Child Adolesc Health. (2018) 2:191–204. 10.1016/S2352-4642(18)30026-9 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

