The Value of ADAMTS13 in Predicting Clinical Outcomes in Patients With Acute Ischemic Stroke Receiving Thrombolysis
- PMID: 32849241
- PMCID: PMC7412597
- DOI: 10.3389/fneur.2020.00799
The Value of ADAMTS13 in Predicting Clinical Outcomes in Patients With Acute Ischemic Stroke Receiving Thrombolysis
Abstract
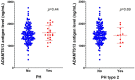
Objective: To determine the association between baseline ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) antigen level and 90-days clinical outcome in patients with acute ischemic stroke (AIS) receiving recombinant tissue plasminogen activator (rt-PA) thrombolysis. Methods: AIS patients receiving rt-PA thrombolytic therapy from Huashan Hospital and Fifth People's Hospital of Shanghai, China in 2014-2017 were consecutively enrolled. Blood samples for ADAMTS13 tests were drawn before intravenous rt-PA administration. The primary outcome was defined as the poor functional outcome of modified Rankin Scale (mRS) >2 at 90-days follow-up. Secondary outcome was hemorrhagic transformation after rt-PA therapy. Moreover, for AIS patients with large vessel occlusion from Huashan Hospital, the association between baseline ADAMTS13 level and cerebral collateral flow was also assessed. Results: A total of 163 AIS patients (median age 66.2 years, 63.8% male) were included. Baseline ADAMTS13 level was marginally decreased in patients with 90-days mRS >2 than in those with mRS ≤ 2 (mean ± SD, 1458.4 ± 323.3 vs. 1578.3 ± 395.4 ng/mL, p = 0.046). However, no difference of ADAMTS13 level was found after adjusting for age, history of atrial fibrillation, glycemia, baseline NIHSS score and TOAST classification (p = 0.43). We found no difference in ADAMTS13 level between patients with parenchymal hemorrhage after rt-PA therapy and those without (p = 0.44). Among 66 patients with large vessel occlusion, there was also no association between ADAMTS13 level and cerebral collateral flow in multivariable analyses. Conclusion: In our cohort, blood ADAMTS13 antigen level before rt-PA therapy could not be used as an independent biomarker in predicting clinical outcomes of AIS patients at 90 days.
Keywords: ADAMTS13; clinical outcome; collateral flow; hemorrhagic transformation; infarct core; ischemic stroke; thrombolysis.
Copyright © 2020 Su, Chen, Ye, Sun, Wu, Dong, Cheng and Wu.
Figures
References
-
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2018) 49:e46–110. 10.1161/STR.0000000000000158 - DOI - PubMed
-
- Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. . Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-australasian acute stroke study investigators. Lancet. (1998) 352:1245–51. 10.1016/S0140-6736(98)08020-9 - DOI - PubMed
LinkOut - more resources
Full Text Sources

