Telomerase Reverse Transcriptase (TERT) Regulation in Thyroid Cancer: A Review
- PMID: 32849278
- PMCID: PMC7412884
- DOI: 10.3389/fendo.2020.00485
Telomerase Reverse Transcriptase (TERT) Regulation in Thyroid Cancer: A Review
Abstract
Telomerase reverse transcriptase (TERT) is the catalytic subunit of the enzyme telomerase and is essential for telomerase activity. Upregulation of TERT expression and resulting telomerase activity occurs in the large majority of malignancies, including thyroid cancer. This upregulation results in continued cellular proliferation and avoidance of cellular senescence and cell death. In this review we will briefly introduce TERT and telomerase activity as it pertains to thyroid cancer and, highlight the effects of TERT on cancer cells. We will also explore in detail the different TERT regulatory strategies and how TERT is reactivated in thyroid cancer cells, specifically. These regulatory mechanisms include both activating single base pair TERT promoter mutations and epigenetic changes at the promoter, including changes in CpG methylation and histone modifications that affect chromatin structure. Further, regulation includes the allele-specific regulation of the TERT promoter in thyroid cancer cells harboring the TERT promoter mutation. These entail allele-specific transcriptional activator binding, DNA methylation, histone modifications, and mono-allelic expression of TERT. Lastly, TERT copy number alterations and alternative splicing are also implicated. Both amplifications of the TERT locus and increased full-length transcripts and decreased inactive and dominant negative isoforms result in active telomerase. Finally, the clinical significance of TERT in thyroid cancer is also reviewed.
Keywords: TERT; alternative splice variants; copy number variation; epigenetics; telomerase; thyroid cancer; transcription.
Copyright © 2020 McKelvey, Umbricht and Zeiger.
Figures
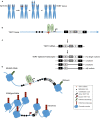
 ) and TERT promoter mutation (
) and TERT promoter mutation ( ), with hypermethylation further upstream of the TSS (
), with hypermethylation further upstream of the TSS ( ). Transcriptional activators MYC and ETS family factors bind at the TERT promoter, the latter binding in the presence of the TERT mutation only. (C) Alternative splicing of TERT in thyroid cancer. TERT is transcribed into mRNA, containing 16 exons (#5–9 shown). TERT can be alternatively spliced, into either the full length, α, β, or α/β isoforms. (D) Allele-specific regulation of the TERT promoter in thyroid cancer. The TERT mutant allele, with mutation (
). Transcriptional activators MYC and ETS family factors bind at the TERT promoter, the latter binding in the presence of the TERT mutation only. (C) Alternative splicing of TERT in thyroid cancer. TERT is transcribed into mRNA, containing 16 exons (#5–9 shown). TERT can be alternatively spliced, into either the full length, α, β, or α/β isoforms. (D) Allele-specific regulation of the TERT promoter in thyroid cancer. The TERT mutant allele, with mutation ( ) is in an open chromatin conformation, with histones spread across the DNA, depicted by the cluster of blue circles. The wildtype allele is in the closed chromatin conformation, with compacted histones on the DNA. The mutant TERT allele is associated with activating H3K4me3 histone marks, represented by the green squares, a CpG unmethylated (◯) promoter and methylated (
) is in an open chromatin conformation, with histones spread across the DNA, depicted by the cluster of blue circles. The wildtype allele is in the closed chromatin conformation, with compacted histones on the DNA. The mutant TERT allele is associated with activating H3K4me3 histone marks, represented by the green squares, a CpG unmethylated (◯) promoter and methylated ( ) gene body. The transcription factors GABPA and MYC bind at the promoter, and TERT is actively transcribed. The wildtype allele is associated with silencing H3K27me3 histone marks, represented by red squares, and a CpG methylated promoter.
) gene body. The transcription factors GABPA and MYC bind at the promoter, and TERT is actively transcribed. The wildtype allele is associated with silencing H3K27me3 histone marks, represented by red squares, and a CpG methylated promoter.References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

