Microbial Profile During Pericoronitis and Microbiota Shift After Treatment
- PMID: 32849467
- PMCID: PMC7422626
- DOI: 10.3389/fmicb.2020.01888
Microbial Profile During Pericoronitis and Microbiota Shift After Treatment
Abstract
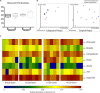
The microflora of the distal pocket is considered as the major cause of pericoronitis. How the oral microflora changes during pericoronitis and whether different types of impacted third molar harbor the same microflora are still unknown. Saliva, subgingival plaque, and gingival plaque of mandibular third molars (M3Ms) were collected from twelve patients with acute pericoronitis. They were given local irrigation or local irrigation + antibiotics according to symptoms. Samples were harvested at the first visit with pericoronitis, 1 week after treatment, and 6 weeks after treatment. 16S rRNA gene polymerase chain reaction products were generated and sequenced after DNA isolation. Comparison of three sampling sites showed that, the subgingival plaque of M3Ms had most remarkable changes in symptomatic period, including a significant increase in microbial richness, and a convergent trend in microbial composition. After treatment, the subgingival microbiome was altered and largely returned to the state in asymptomatic period. In summary, the distal subgingival microbiota of M3M was most likely to be associated with the pathogenesis of pericoronitis. The post-treatment microbiota shift of M3M proved the effectiveness of treatment. The inclination type of impacted M3Ms and treatment method would also make a difference to the pericoronal microbiota.
Keywords: 16S rRNA; microbiome; pericoronitis; sequencing; treatment.
Copyright © 2020 Huang, Zheng, An, Chen, Xiao and Zhang.
Figures



References
LinkOut - more resources
Full Text Sources

