Functional Reciprocity of Amyloids and Antimicrobial Peptides: Rethinking the Role of Supramolecular Assembly in Host Defense, Immune Activation, and Inflammation
- PMID: 32849553
- PMCID: PMC7412598
- DOI: 10.3389/fimmu.2020.01629
Functional Reciprocity of Amyloids and Antimicrobial Peptides: Rethinking the Role of Supramolecular Assembly in Host Defense, Immune Activation, and Inflammation
Abstract
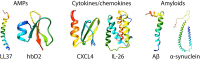
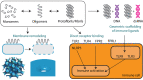
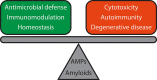
Pathological self-assembly is a concept that is classically associated with amyloids, such as amyloid-β (Aβ) in Alzheimer's disease and α-synuclein in Parkinson's disease. In prokaryotic organisms, amyloids are assembled extracellularly in a similar fashion to human amyloids. Pathogenicity of amyloids is attributed to their ability to transform into several distinct structural states that reflect their downstream biological consequences. While the oligomeric forms of amyloids are thought to be responsible for their cytotoxicity via membrane permeation, their fibrillar conformations are known to interact with the innate immune system to induce inflammation. Furthermore, both eukaryotic and prokaryotic amyloids can self-assemble into molecular chaperones to bind nucleic acids, enabling amplification of Toll-like receptor (TLR) signaling. Recent work has shown that antimicrobial peptides (AMPs) follow a strikingly similar paradigm. Previously, AMPs were thought of as peptides with the primary function of permeating microbial membranes. Consistent with this, many AMPs are facially amphiphilic and can facilitate membrane remodeling processes such as pore formation and fusion. We show that various AMPs and chemokines can also chaperone and organize immune ligands into amyloid-like ordered supramolecular structures that are geometrically optimized for binding to TLRs, thereby amplifying immune signaling. The ability of amphiphilic AMPs to self-assemble cooperatively into superhelical protofibrils that form structural scaffolds for the ordered presentation of immune ligands like DNA and dsRNA is central to inflammation. It is interesting to explore the notion that the assembly of AMP protofibrils may be analogous to that of amyloid aggregates. Coming full circle, recent work has suggested that Aβ and other amyloids also have AMP-like antimicrobial functions. The emerging perspective is one in which assembly affords a more finely calibrated system of recognition and response: the detection of single immune ligands, immune ligands bound to AMPs, and immune ligands spatially organized to varying degrees by AMPs, result in different immunologic outcomes. In this framework, not all ordered structures generated during multi-stepped AMP (or amyloid) assembly are pathological in origin. Supramolecular structures formed during this process serve as signatures to the innate immune system to orchestrate immune amplification in a proportional, situation-dependent manner.
Keywords: Toll-like receptors; amyloids; antimicrobial peptides; autoimmune diseases; innate immunity; neurodegenerative diseases; self-assembly.
Copyright © 2020 Lee, Srinivasan, de Anda, Nicastro, Tükel and Wong.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

