Supraphysiological Levels of Testosterone Induce Vascular Dysfunction via Activation of the NLRP3 Inflammasome
- PMID: 32849566
- PMCID: PMC7411079
- DOI: 10.3389/fimmu.2020.01647
Supraphysiological Levels of Testosterone Induce Vascular Dysfunction via Activation of the NLRP3 Inflammasome
Abstract
Background: Both supraphysiological and subphysiological testosterone levels are associated with increased cardiovascular risk. Testosterone consumption at supraphysiological doses has been linked to increased blood pressure, left ventricular hypertrophy, vascular dysfunction, and increased levels of inflammatory markers. Activation of the NLRP3 inflammasome contributes to the production of proinflammatory cytokines, leading to cardiovascular dysfunction. We hypothesized that supraphysiological levels of testosterone, via generation of mitochondrial reactive oxygen species (mROS), activates the NLRP3 inflammasome and promotes vascular dysfunction. Methods: Male, 12 week-old C57Bl/6J (WT) and NLRP3 knockout (NLRP3-/-) mice were used. Mice were treated with testosterone propionate [TP (10 mg/kg) in vivo] or vehicle for 30 days. In addition, vessels were incubated with testosterone [Testo (10-6 M, 2 h) in vitro]. Testosterone levels, blood pressure, vascular function (thoracic aortic rings), pro-caspase-1/caspase-1 and interleukin-1β (IL-1β) expression, and generation of reactive oxygen species were determined. Results: Testosterone increased contractile responses and reduced endothelium-dependent vasodilation, both in vivo and in vitro. These effects were not observed in arteries from NLRP3-/- mice. Aortas of TP-treated WT mice (in vivo), as well as aortas from WT mice incubated with testo (in vitro), exhibited increased mROS levels and increased caspase-1 and IL-1β expression. These effects were not observed in arteries from NLRP3-/- mice. Flutamide [Flu, 10-5 M, androgen receptor (AR) antagonist], carbonyl cyanide m-chlorophenyl hydrazone (CCCP, 10-6 M, mitochondrial uncoupler) and MCC950 (MCC950, 10-6 M, a NLRP3 receptor inhibitor) prevented testosterone-induced mROS generation. Conclusion: Supraphysiological levels of testosterone induce vascular dysfunction via mROS generation and NLRP3 inflammasome activation. These events may contribute to increased cardiovascular risk.
Keywords: NLRP3 inflammasome; androgen receptor; reactive oxygen species; testosterone; vascular dysfunction.
Copyright © 2020 Alves, da Costa, Pereira, Fedoce, Silva, Carneiro, Lobato and Tostes.
Figures
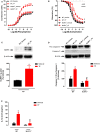
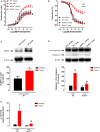
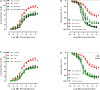
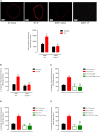

Comment in
-
Male and Female Sexual Function and Dysfunction; Andrology.J Urol. 2021 Apr;205(4):1208-1210. doi: 10.1097/JU.0000000000001618. Epub 2021 Jan 19. J Urol. 2021. PMID: 33464933 No abstract available.
References
-
- George J, Rapsomaniki E, Pujades-Rodriguez M, Shah AD, Denaxas S, Herrett E, et al. . How does cardiovascular disease first present in women and men? Incidence of 12 cardiovascular diseases in a contemporary cohort of 1 937 360 people. Circulation. (2015) 132:1320–8. 10.1161/CIRCULATIONAHA.114.013797 - DOI - PMC - PubMed
-
- Groban L, Lindsey SH, Wang H, Alencar AK. Sex and gender differences in cardiovascular disease. Sex Diff Physiol. (2016) 1:61–87. 10.1016/B978-0-12-802388-4.00005-7 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

