Eosinophilia Associated With CD3-CD4+ T Cells: Characterization and Outcome of a Single-Center Cohort of 26 Patients
- PMID: 32849632
- PMCID: PMC7432433
- DOI: 10.3389/fimmu.2020.01765
Eosinophilia Associated With CD3-CD4+ T Cells: Characterization and Outcome of a Single-Center Cohort of 26 Patients
Abstract
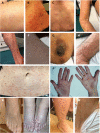
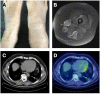
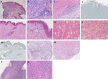
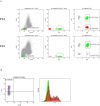
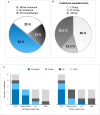
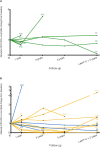
Background: Lymphocytic variant hypereosinophilic syndrome is characterized by marked over-production of eosinophilopoietic factor(s) by dysregulated T cells leading to eosinophil expansion. In most cases, these T cells are clonal and express a CD3-CD4+ phenotype. As this is a rare disorder, presenting manifestations, disease course, treatment responses, and outcome are not well-characterized. Materials and Methods: In this retrospective single-center observational study, we reviewed medical files of all patients with persistent hypereosinophilia seen between 1994 and 2019 in whom CD3-CD4+ T cells were detected. Data collection included clinical and biological findings at presentation, treatment responses, disease course, and serial CD3-CD4+ T cell counts. Results: Our cohort comprises 26 patients, including 2 with hypereosinophilia of undetermined significance. All 24 symptomatic patients had cutaneous lesions and/or angioedema, and fasciitis was present in several cases. The aberrant T cell subset represented 2% or less total lymphocytes in 11 subjects. TCR gene rearrangement patterns on whole blood were polyclonal in these cases, while they all had serum CCL17/TARC levels above 1,500 pg/ml. Disease manifestations were mild and did not require maintenance therapy in roughly one third of the cohort, while two thirds required long-term oral corticosteroids and/or second-line agents. Among these, interferon-alpha was the most effective treatment option with a response observed in 8/8 patients, one of whom was cured of disease. Treatment had to be interrupted in most cases however due to poor tolerance and/or development of secondary resistance. Anti-interleukin-5 antibodies reduced blood eosinophilia in 5/5 patients, but clinical responses were disappointing. A sub-group of 5 patients had severe treatment-refractory disease, and experienced significant disease- and treatment-related morbidity and mortality, including progression to T cell lymphoma in three. Conclusions: This retrospective longitudinal analysis of the largest monocentric cohort of CD3-CD4+ T cell associated lymphocytic variant hypereosinophilic syndrome published so far provides clinicians confronted with this rare disorder with relevant new data on patient presentation and outcome that should help tailor therapy and follow-up to different levels of disease severity. It highlights the need for novel therapeutic options, especially for the subset of patients with severe treatment-refractory disease. Future research efforts should be made toward understanding CD3-CD4+ T cell biology in order to develop new treatments that target primary pathogenic mechanisms.
Keywords: CD3−CD4+ T cells; T cell lymphoma; TARC (CCL17); anti-interleukin-5; fasciitis; interferon-alpha; interleukin-5; lymphocytic variant hypereosinophilic syndrome.
Copyright © 2020 Carpentier, Verbanck, Schandené, Heimann, Trépant, Cogan and Roufosse.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

