Neutrophils: Orchestrators of the Malignant Phenotype
- PMID: 32849639
- PMCID: PMC7433712
- DOI: 10.3389/fimmu.2020.01778
Neutrophils: Orchestrators of the Malignant Phenotype
Abstract
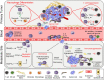
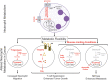
Neutrophils are the first leukocytes recruited to sites of inflammation, where they execute anti-microbial functions to eliminate infectious agents. These functions include phagocytosis, release of reactive oxygen species and the formation of neutrophil extracellular traps via NETosis. Neutrophils are receiving increasing attention in the context of cancer, where these same neutrophil-associated functions are also important for modulating tumor growth and metastatic progression. Neutrophils are phenotypically heterogeneous and, depending on the context, exert anti- or pro-tumorigenic functions. Increasing evidence also suggests an important role of neutrophils and their involvement in promoting multiple steps of the metastatic cascade. The steps include: (1) local invasion and intravasation of cancer cells into circulation, (2) survival of cancer cells in the bloodstream and extravasation at a distant site, (3) early cancer cell seeding/survival, and (4) progressive growth of cancer cells to form macroscopic metastases. Although neutrophil functions designed to eliminate infectious agents can also eliminate tumor cells, their dysregulation can promote tumor growth and enable metastasis at multiple steps along the metastatic cascade. In this review, we will provide an overview of the current advances in neutrophil biology in the context of cancer. We also discuss the emerging field of immunometabolism, in which the rewiring of alternative metabolic pathways within neutrophils can impact their pro-tumorigenic/pro-metastatic functions.
Keywords: NETosis; immunometabolism; immunosuppression; metabolic plasticity/flexibility; metastasis; neutrophils; tumor growth.
Copyright © 2020 Hsu, Shen and Siegel.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

