Longer Duration of SARS-CoV-2 Infection in a Case of Mild COVID-19 With Weak Production of the Specific IgM and IgG Antibodies
- PMID: 32849650
- PMCID: PMC7426437
- DOI: 10.3389/fimmu.2020.01936
Longer Duration of SARS-CoV-2 Infection in a Case of Mild COVID-19 With Weak Production of the Specific IgM and IgG Antibodies
Abstract
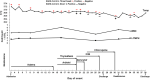
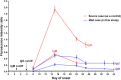
Background: The relationship between SARS-CoV-2-carrying time and specific antibody production has not yet been reported in re-admitted COVID-19 patients. We reported a case of mild COVID-19 with long virus-carrying time, weak production of virus-specific IgG and IgM antibodies, and recurrence of positive SARS-CoV-2 RNA in stool specimens after discharge. Case Presentation: A 27-year-old male was diagnosed as COVID-19 after returning to Meizhou from Wuhan. Despite extremely mild symptoms, the patient was hospitalized for 24 days because of persistent positive SARS-CoV-2 RNA detection. Three days after recovery discharge, he was hospitalized again for 7 days due to a recurrence of the positive SARS-CoV-2 RNA result, while in a good physical condition. Serological assay, using a fluorescent immunochromatography detection kit specific to SARS-CoV-2, showed that SARS-CoV-2-specific IgM antibodies were undetectable and IgG antibodies were very low on day 8 after onset; both of the antibodies seemingly reached top concentrations on day 15 (just a 6-fold increase of the IgG titer), and then decreased, remaining relatively stable from day 25 after onset until discharge. The production of the IgM and IgG targeting SARS-CoV-2 in this very mild case was much lower than that in a severe case of COVID-19 during the same hospitalizing period, and the latter was used as a control. Conclusion: Mild COVID-19 patients could carry SARS-CoV-2 for a long time, which may be related to the weak production of the virus-specific IgG and IgM. Recurrence of positive SARS-CoV-2 RNA could occur in mild COVID-19 possibly due to intermittent virus shedding, so strict quarantine and health surveillance should be taken for all discharged COVID-19 patients to prevent a potential virus spread.
Keywords: SARS-CoV-2; case report; mild COVID-19; persistent infection; virus-specific antibody response.
Copyright © 2020 Guo, Zeng, Huang, He, Zhang and Zhong.
Figures
Similar articles
-
Antibody kinetics and serologic profiles of SARS-CoV-2 infection using two serologic assays.PLoS One. 2020 Oct 22;15(10):e0240395. doi: 10.1371/journal.pone.0240395. eCollection 2020. PLoS One. 2020. PMID: 33091042 Free PMC article.
-
Value of Viral Nucleic Acid in Sputum and Feces and Specific IgM/IgG in Serum for the Diagnosis of Coronavirus Disease 2019.Front Cell Infect Microbiol. 2020 Aug 6;10:445. doi: 10.3389/fcimb.2020.00445. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32850506 Free PMC article.
-
Antibody responses to SARS-CoV-2 in patients with differing severities of coronavirus disease 2019.PLoS One. 2020 Oct 9;15(10):e0240502. doi: 10.1371/journal.pone.0240502. eCollection 2020. PLoS One. 2020. PMID: 33035234 Free PMC article.
-
Value and Validity of Coronavirus Antibody Testing.Pain Physician. 2020 Aug;23(4S):S381-S390. Pain Physician. 2020. PMID: 32942795 Review.
-
Urinary Viral Shedding of COVID-19 and its Clinical Associations: A Systematic Review and Meta-analysis of Observational Studies.Urol J. 2020 Sep 5;17(5):433-441. doi: 10.22037/uj.v16i7.6248. Urol J. 2020. PMID: 32888186
Cited by
-
Cross-linking peptide and repurposed drugs inhibit both entry pathways of SARS-CoV-2.Nat Commun. 2021 Mar 9;12(1):1517. doi: 10.1038/s41467-021-21825-w. Nat Commun. 2021. PMID: 33750821 Free PMC article.
-
The Utility of Specific Antibodies Against SARS-CoV-2 in Laboratory Diagnosis.Front Microbiol. 2021 Jan 13;11:603058. doi: 10.3389/fmicb.2020.603058. eCollection 2020. Front Microbiol. 2021. PMID: 33519745 Free PMC article. Review.
-
Predictive value of immunoglobulin G, activated partial thromboplastin time, platelet, and indirect bilirubin for delayed viral clearance in patients infected with the Omicron variant.PeerJ. 2023 May 19;11:e15443. doi: 10.7717/peerj.15443. eCollection 2023. PeerJ. 2023. PMID: 37223120 Free PMC article.
-
Recurrence, Reactivation, or Inflammatory Rebound of SARS-CoV-2 Infection With Acute Vestibular Symptoms: A Case Report and Revision of Literature.Front Hum Neurosci. 2021 Aug 11;15:666468. doi: 10.3389/fnhum.2021.666468. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34456694 Free PMC article.
-
SARS-CoV-2-induced Overexpression of miR-4485 Suppresses Osteogenic Differentiation and Impairs Fracture Healing.Int J Biol Sci. 2021 Mar 25;17(5):1277-1288. doi: 10.7150/ijbs.56657. eCollection 2021. Int J Biol Sci. 2021. PMID: 33867845 Free PMC article.
References
-
- General Office of National Health Commission General Office of National Administration of Traditional Chinese Medicine Diagnostic and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). (2020). Available online at: http://www.gov.cn/zhengce/zhengceku/2020-03/04/5486705/files/ae61004f930... (accessed April 27, 2020).
-
- World Health Organization . Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). (2020). Available online at: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mis... (accessed April 27, 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

