Immunoglobulin M: An Ancient Antiviral Weapon - Rediscovered
- PMID: 32849652
- PMCID: PMC7432194
- DOI: 10.3389/fimmu.2020.01943
Immunoglobulin M: An Ancient Antiviral Weapon - Rediscovered
Abstract
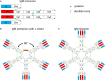
Recent discoveries have shed new light onto immunoglobulin M (IgM), an ancient antibody class preserved throughout evolution in all vertebrates. First, IgM - long thought to be a perfect pentamer - was shown to be asymmetric, resembling a quasi-hexamer missing one monomer and containing a gap. Second, this gap allows IgM to serve as carrier of a specific host protein, apoptosis inhibitor of macrophages (AIM), which is released to promote removal of dead-cell debris, cancer cells, or pathogens. Third, recombinant IgM delivered mucosally by passive immunization gave proof-of-concept that this antibody class can prevent mucosal simian-human immunodeficiency virus transmission in non-human primates. Finally, IgM's role in adaptive immunity goes beyond being only a first defender to respond to pathogen invasion, as long-lived IgM plasma cells have been observed predominantly residing in the spleen. In fact, IgM produced by such cells contained somatic hypermutations and was linked to protection against lethal influenza virus challenge in murine models. Importantly, such long-lived IgM plasma cells had been induced by immunization 1 year before challenge. Together, new data on IgM function raise the possibility that vaccine strategies aimed at preventing virus acquisition could include this ancient weapon.
Keywords: IgM function; IgM structure; passive mucosal immunization with IgM; prevention of mucosal virus transmission by IgM; recombinant monoclonal IgM; vaccine-induced long-lived IgM plasma cells.
Copyright © 2020 Gong and Ruprecht.
Figures
References
-
- Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. (2008) 151:42–50. 10.1111/j.1365-2249.2007.03545.x - DOI - PMC - PubMed
-
- Janeway CA, Jr., Travers P, Walport M, Shlomchik MJ. Structural variation in immunoglobulin constant regions. 5th ed In: Janeway C. editor. Immunobiology: The Immune System in Health and Disease. New York, NY: Garland Science; (2001).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

