Rice OsLHT1 Functions in Leaf-to-Panicle Nitrogen Allocation for Grain Yield and Quality
- PMID: 32849708
- PMCID: PMC7403224
- DOI: 10.3389/fpls.2020.01150
Rice OsLHT1 Functions in Leaf-to-Panicle Nitrogen Allocation for Grain Yield and Quality
Abstract
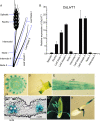
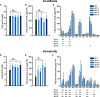
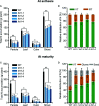
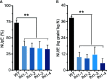
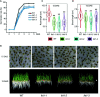
Proper allocation of nitrogen (N) from source leaves to grains is essential step for high crop grain yield and N use efficiency. In rice (Oryza sativa) grown in flooding paddy field, amino acids are the major N compounds for N distribution and re-allocation. We have recently identified that Lysine-Histidine-type Transporter 1 (OsLHT1) is the major transporter for root uptake and root-to-shoot allocation of amino acids in rice. In this study, we planted knockout mutant lines of OsLHT1 together wild-type (WT) in paddy field for evaluating OsLHT1 function in N redistribution and grain production. OsLHT1 is expressed in vascular bundles of leaves, rachis, and flowering organs. Oslht1 plants showed lower panicle length and seed setting rate, especially lower grain number per panicle and total grain weight. The concentrations of both total N and free amino acids in the flag leaf were similar at anthesis between Oslht1 lines and WT while significantly higher in the mutants than WT at maturation. The Oslht1 seeds contained higher proteins and most of the essential free amino acids, similar total starch but less amylose with lower paste viscosity than WT seeds. The mutant seeds showed lower germination rate than WT. Knockout of OsLHT1 decreased N uptake efficiency and physiological utilization efficiency (kg-grains/kg-N) by about 55% and 72%, respectively. Taken together, we conclude that OsLHT1 plays critical role in the translocation of amino acids from vegetative to reproductive organs for grain yield and quality of nutrition and functionality.
Keywords: OsLHT1; amino acids; grain quality; grain yield; nitrogen allocation; rice; transporter.
Copyright © 2020 Guo, Gu, Hu, Qu and Xu.
Figures


References
-
- Ambika S., Manonmani V., Somasundar G. (2014). Review on effect of seed size on seedling vigour and seed yield. Res. J. Seed Sci. 7 (2), 31–38. 10.3923/rjss.2014.31.38 - DOI
-
- Balasubramanian V., Alves B., Aulakh M., Bekunda M., Cai Z., Drinkwater L., et al. (2004). Crop, environmental, and management factors affecting nitrogen use efficiency. Agriculture and the Nitrogen Cycle: Assessing the impacts of fertilizer use on food production and the environment. SCOPE; (65), 19–33.
LinkOut - more resources
Full Text Sources

