Advanced Microscopy Reveals Complex Developmental and Subcellular Localization Patterns of ANNEXIN 1 in Arabidopsis
- PMID: 32849711
- PMCID: PMC7419693
- DOI: 10.3389/fpls.2020.01153
Advanced Microscopy Reveals Complex Developmental and Subcellular Localization Patterns of ANNEXIN 1 in Arabidopsis
Abstract
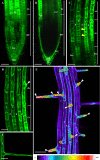
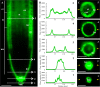
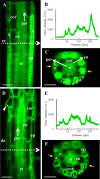
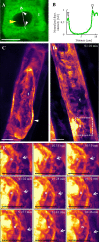
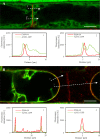
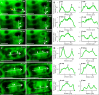
Annexin 1 (ANN1) is the most abundant member of the evolutionary conserved multigene protein superfamily of annexins in plants. Generally, annexins participate in diverse cellular processes, such as cell growth, differentiation, vesicle trafficking, and stress responses. The expression of annexins is developmentally regulated, and it is sensitive to the external environment. ANN1 is expressed in almost all Arabidopsis tissues, while the most abundant is in the root, root hairs, and in the hypocotyl epidermal cells. Annexins were also occasionally proposed to associate with cytoskeleton and vesicles, but they were never developmentally localized at the subcellular level in diverse plant tissues and organs. Using advanced light-sheet fluorescence microscopy (LSFM), we followed the developmental and subcellular localization of GFP-tagged ANN1 in post-embryonic Arabidopsis organs. By contrast to conventional microscopy, LSFM allowed long-term imaging of ANN1-GFP in Arabidopsis plants at near-environmental conditions without affecting plant viability. We studied developmental regulation of ANN1-GFP expression and localization in growing Arabidopsis roots: strong accumulation was found in the root cap and epidermal cells (preferentially in elongating trichoblasts), but it was depleted in dividing cells localized in deeper layers of the root meristem. During root hair development, ANN1-GFP accumulated at the tips of emerging and growing root hairs, which was accompanied by decreased abundance in the trichoblasts. In aerial plant parts, ANN1-GFP was localized mainly in the cortical cytoplasm of trichomes and epidermal cells of hypocotyls, cotyledons, true leaves, and their petioles. At the subcellular level, ANN1-GFP was enriched at the plasma membrane (PM) and vesicles of non-dividing cells and in mitotic and cytokinetic microtubular arrays of dividing cells. Additionally, an independent immunolocalization method confirmed ANN1-GFP association with mitotic and cytokinetic microtubules (PPBs and phragmoplasts) in dividing cells of the lateral root cap. Lattice LSFM revealed subcellular accumulation of ANN1-GFP around the nuclear envelope of elongating trichoblasts. Massive relocation and accumulation of ANN1-GFP at the PM and in Hechtian strands and reticulum in plasmolyzed cells suggest a possible osmoprotective role of ANN1-GFP during plasmolysis/deplasmolysis cycle. This study shows complex developmental and subcellular localization patterns of ANN1 in living Arabidopsis plants.
Keywords: Arabidopsis thaliana; annexin 1; cell division; development; lattice light-sheet fluorescence microscopy; light-sheet fluorescence microscopy; plasma membrane; subcellular localization.
Copyright © 2020 Tichá, Richter, Ovečka, Maghelli, Hrbáčková, Dvořák, Šamaj and Šamajová.
Figures




References
-
- Blackbourn H. D., Battey N. H. (1993). Annexin-mediated secretory vesicle aggregation in plants. Physiol. Plant 89, 27–32. 10.1111/j.1399-3054.1993.tb01782.x - DOI
-
- Brown R. M. (2004). Cellulose structure and biosynthesis: What is in store for the 21st century? J. Polym. Sci. A Polym. Chem. 42, 487–495. 10.1002/pola.10877 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

