Evaluation of Hemodynamic Change by Indocyanine Green-FLOW 800 Videoangiography Mapping: Prediction of Hyperperfusion Syndrome in Patients with Moyamoya Disease
- PMID: 32850003
- PMCID: PMC7441439
- DOI: 10.1155/2020/8561609
Evaluation of Hemodynamic Change by Indocyanine Green-FLOW 800 Videoangiography Mapping: Prediction of Hyperperfusion Syndrome in Patients with Moyamoya Disease
Abstract
Objective: Hyperperfusion syndrome (HPS) after bypass surgery for moyamoya disease (MMD) mainly results from redistribution of blood flow, which leads to poor outcomes, while effective methods to predict HPS are still lacking. Indocyanine green (ICG) videoangiography can assess regional cerebral blood flow changes semiquantitatively with the application of FLOW 800 software. The purpose of this study was to investigate whether the intraoperative evaluation of local hemodynamic changes around anastomotic sites using FLOW 800 videoangiography mapping can predict the incidence of HPS and clinical outcomes.
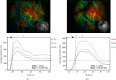
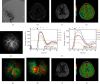
Methods: Of the patients who were diagnosed with MMD in our hospital between August 2018 and December 2019, who underwent superficial temporal artery-middle cerebral artery bypass surgeries, we investigated 65 hemispheres (in 62 patients) in which intraoperative ICG analysis was performed using FLOW 800 (Zeiss Meditec, Oberkochen, Germany) to evaluate the local cerebral hemodynamics before and after anastomosis. Regions of interest were set at more than 2 points on the brain surface according to the location and situation of recipient arteries in the surgical area. Peak cerebral blood volume (CBV), regional cerebral blood flow (CBF), and time to peak (TTP) were calculated from the selected points. As the data were available intraoperatively, anastomoses were performed in a suitable area. According to the occurrence of HPS, patients were divided into the asymptomatic and symptomatic groups, from which hemodynamic parameters were compared. Furthermore, ROC analysis was performed to determine the diagnostic accuracy of change rates in CBV, CBF, and TTP (i.e., ΔCBV, ΔCBF, and ΔTTP) for predicting HPS.
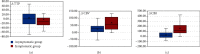
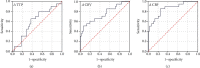
Results: Data from the 62 patients were analyzed, and all patients were closely assessed during hospitalization after the procedures. The values of ΔCBV and ΔCBF were significantly higher in the symptomatic group (p < 0.01), while ΔTTP is slightly lower in the symptomatic group with no statistical differences (p = 0.72). Hemodynamic parameters including ΔCBV and ΔCBF, calculated by FLOW 800, had high sensitivity and specificity according to the ROC curve (ΔCBV: AUC = 0.743, 95% CI, 0.605-0.881, p = 0.002; ΔCBF: AUC = 0.852, 95% CI, 0.750-0.954, p < 0.01), which could be used as predictors for HPS.
Conclusions: Intraoperative ICG-FLOW 800 videoangiography mapping is a safe method which can reflect hemodynamic characteristics in the surgical area for MMD, the findings of which correlate with the occurrence of HPS. Parameters including ΔCBV and ΔCBF are proven to be efficient in the prediction of HPS.
Copyright © 2020 Xin Zhang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
The value of indocyanine green-FLOW800 in microvasculature for predicting cerebral hyperperfusion syndrome in moyamoya disease patients.Sci Rep. 2023 Oct 26;13(1):18352. doi: 10.1038/s41598-023-45676-1. Sci Rep. 2023. PMID: 37884669 Free PMC article.
-
Intraoperative evaluation of local cerebral hemodynamic change by indocyanine green videoangiography: prediction of incidence and duration of postoperative transient neurological events in patients with moyamoya disease.J Neurosurg. 2018 Apr 20;130(4):1367-1375. doi: 10.3171/2017.10.JNS171523. Print 2019 Apr 1. J Neurosurg. 2018. PMID: 29676693
-
Correlation between reduction in microvascular transit time after superficial temporal artery-middle cerebral artery bypass surgery for moyamoya disease and the development of postoperative hyperperfusion syndrome.J Neurosurg. 2018 May;128(5):1304-1310. doi: 10.3171/2016.11.JNS162403. Epub 2017 May 12. J Neurosurg. 2018. PMID: 28498060
-
Intraoperative assessment of cortical perfusion by indocyanine green videoangiography in surgical revascularization for moyamoya disease.Acta Neurochir (Wien). 2014 Sep;156(9):1753-60. doi: 10.1007/s00701-014-2161-2. Epub 2014 Jun 28. Acta Neurochir (Wien). 2014. PMID: 24973201
-
Progress on Complications of Direct Bypass for Moyamoya Disease.Int J Med Sci. 2016 Jul 5;13(8):578-87. doi: 10.7150/ijms.15390. eCollection 2016. Int J Med Sci. 2016. PMID: 27499690 Free PMC article. Review.
Cited by
-
Multimodal evaluation of the bloodstream alteration before and after combined revascularization for Moyamoya disease.Front Neurol. 2023 Sep 15;14:1249914. doi: 10.3389/fneur.2023.1249914. eCollection 2023. Front Neurol. 2023. PMID: 37780715 Free PMC article.
-
Application of intraoperative infrared thermography in bypass surgery for adult moyamoya syndrome: A preliminary study.Front Neurol. 2023 Mar 30;14:1174072. doi: 10.3389/fneur.2023.1174072. eCollection 2023. Front Neurol. 2023. PMID: 37064202 Free PMC article.
-
The value of indocyanine green-FLOW800 in microvasculature for predicting cerebral hyperperfusion syndrome in moyamoya disease patients.Sci Rep. 2023 Oct 26;13(1):18352. doi: 10.1038/s41598-023-45676-1. Sci Rep. 2023. PMID: 37884669 Free PMC article.
-
Combination of intraoperative indocyanine green video-angiography FLOW 800 and computed tomography perfusion to assess the risk of cerebral hyperperfusion syndrome in chronic internal carotid artery occlusion patients after revascularization surgery.Front Neurol. 2023 Dec 5;14:1323626. doi: 10.3389/fneur.2023.1323626. eCollection 2023. Front Neurol. 2023. PMID: 38125835 Free PMC article.
-
Perfusion Parameters in Near-Infrared Fluorescence Imaging with Indocyanine Green: A Systematic Review of the Literature.Life (Basel). 2021 May 11;11(5):433. doi: 10.3390/life11050433. Life (Basel). 2021. PMID: 34064948 Free PMC article. Review.
References
-
- Uda K., Araki Y., Muraoka S., et al. Intraoperative evaluation of local cerebral hemodynamic change by indocyanine green videoangiography: prediction of incidence and duration of postoperative transient neurological events in patients with moyamoya disease. Journal of Neurosurgery. 2018:1–9. doi: 10.3171/2017.10.JNS171523. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

