Complications of leadless vs conventional (lead) artificial pacemakers - a retrospective review
- PMID: 32850090
- PMCID: PMC7427453
- DOI: 10.1080/20009666.2020.1786901
Complications of leadless vs conventional (lead) artificial pacemakers - a retrospective review
Abstract
Background: Leadless pacemakers (LPM) are introduced in cardiovascular market with a goal to avoid lead- and pocket-associated complications due to conventional artificial pacemakers (CPM). The comparison of LPM and CPM complications is not well studied at a case by case level.
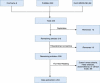
Methods: Comprehensive literature was searched on multiple databases performed from inception to December 2019 and revealed 204 cases that received LPM with a comparison of CPM. The data of complications were extracted, screened by independent authors and analyzed using IBM SPSS Statistics for Windows, Version 22.0 (Armonk, NY: IBM Corp.).
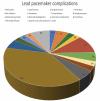
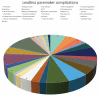
Results: The complications of CPM were high in comparison to LPM in terms of electrode dislodgement (56% vs 7% of cases, p-value < .0001), pocket site infection rate (16% vs 3.4%, p-value = 0.02), and a lead fracture rate (8% vs 0%, p-value = 0.04). LPMs had a statistically non-significant two-times high risk of pericardial effusion (8%) compared to CPMs (4%) with a p-value = 0.8.
Conclusion: LPMs appear to have a better safety profile than CPMs. There was a low pocket site and lead-related infections in LPM as compared to CPM. However, LPM can have twice the risk of pericardial effusion than CPMs, but this was not statistically significant.
Keywords: Conventional pacemaker; Micra transcatheter pacing system (Medtronic); Nanostim® leadless cardiac pacemaker; St. Jude Medical; St. Paul; leadless pacemaker; traditional pacemaker.
© 2020 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group on behalf of Greater Baltimore Medical Center.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

References
-
- Mond HG, Proclemer A.. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009–a world society of arrhythmia’s project. Pacing Clin Electrophysiol. 2011. August;34(8):1013–1027. - PubMed
-
- Greenspon AJ, Patel JD, Lau E, et al. Trends in permanent pacemaker implantation in the USA from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012. October 16;60(16):1540–1545. - PubMed
-
- Ranasinghe I, Parzynski CS, Freeman JV, et al. Long-term risk for device-related complications and reoperations after implantable cardioverter-defibrillator implantation: an observational cohort study. Ann Intern Med. 2016. May 3;165:20. - PubMed
-
- Tarakji KG, Wazni OM, Harb S, et al. Risk factors for 1-year mortality among patients with cardiac implantable electronic device infection undergoing transvenous lead extraction: the impact of the infection type and the presence of vegetation on survival. Europace. 2014. October;16(10):1490–1495. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
