Evaluation of EpiProtect® microbial cellulose burns dressings in young children
- PMID: 32850135
- PMCID: PMC7425250
- DOI: 10.1177/2059513120940503
Evaluation of EpiProtect® microbial cellulose burns dressings in young children
Abstract
Introduction: EpiProtect® is a biosynthetic cellulose dressing indicated for the treatment of superficial burns and the dressing of deep burns. Prior to this study the youngest reported patient treated with EpiProtect® was aged 13 years.
Method: Data were collected prospectively for patients aged < 5 years, presenting to the Children's Burns Unit with ⩾ 2% total body surface area (TBSA) burns sustained by any mechanism.
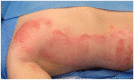
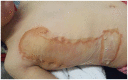
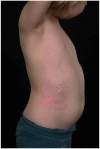
Results: Thirty children were treated (median age = 17 months, age range = 1-61 months). Thirty-six burn depths were documented: superficial partial thickness (SPT) in 53% (n=19); mid-partial thickness (MPT) in 33% (n=12); deep partial thickness (DPT) in 11% (n=4); and full thickness (FT) in 3% (n=1). Median burn size was 4.5% TBSA (range = 2%-12%). EpiProtect® was applied under general anaesthesia in all cases. The median length of stay (LOS) was two days (range = 0-6 days). EpiProtect® was tolerated well and provided effective analgesia for subsequent dressing changes. Median healing time was 13 days (SPT burns), 14 days (MPT) and 24 days (DPT burns). Three patients required split skin grafting. Hypertrophic scarring arose in one patient.
Discussion: This case series represents the youngest published patient group to have been treated with EpiProtect®. Authors conclude that EpiProtect® provides a safe, reliable and well-tolerated dressing option for all burn depths in young children. Importantly, EpiProtect® is culturally neutral and may be used in situations which, for cultural reasons, may preclude the use of animal-derived products. Further studies are warranted to evaluate pain scores, burn depth, size and LOS correlation, and comparative analysis between dressing types.
Lay summary: Burn injuries in the paediatric population are common and often require multiple dressing changes. Dressing changes can be painful and distressing to both children and their care givers. This article describes the experience of using a synthetically derived burns dressing, called EpiProtect®, in children aged ⩽ 5 years. Thirty patients were recruited with varying depths of scald burns and all underwent application of EpiProtect® dressing. The results suggested that EpiProtect® was a user-friendly dressing that can be used to treat partial-thickness burns and to dress full-thickness (FT) burns. It was well-tolerated and provided effective analgesia at the time of dressing changes. There was no incidence of increased burn wound infection rates and all wounds healed. In addition, as EpiProtect® is a synthetic product, it has the benefit of being culturally neutral, which is advantageous in a culturally diverse population. Further studies are warranted to evaluate the effectiveness of this dressing and to compare it to similar dressings that are available.
Keywords: EpiProtect®; Microbial cellulose dressing; full thickness burn; paediatric burns; partial thickness burn; scald; skin substitute.
© The Author(s) 2020.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


References
-
- Kemp A, Jones S, Lawson Z, et al. Patterns of burns and scalds in children. Arch Dis Child 2014; 99: 316–321. - PubMed
-
- Highton L, Wallace C, Shah M. Use of Suprathel® for partial thickness burns in children. Burns 2013; 39(1): 136–141. - PubMed
-
- Everett M, Massand S, Davis W, et al. Use of a copolymer dressing on superficial and partial thickness burns in a paediatric population. J Wound Care 2015; 24(7): S4–S8. - PubMed
-
- Schwarze H, Küntscher M, Uhlig C, et al. Suprathel, a new skin substitute, in the Management of partial-thickness Burn wounds: results of a clinical study. Ann Plast Surg 2008; 60(2): 181–185. - PubMed
LinkOut - more resources
Full Text Sources

