Randomized Controlled Study Comparing Efficacy and Toxicity of Weekly vs. 3-Weekly Induction Chemotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma
- PMID: 32850394
- PMCID: PMC7424062
- DOI: 10.3389/fonc.2020.01284
Randomized Controlled Study Comparing Efficacy and Toxicity of Weekly vs. 3-Weekly Induction Chemotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma
Abstract
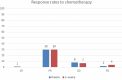
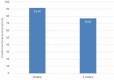
Background: Head and Neck Cancer is a major public health problem in India, majority of which are lifestyle related, male predominant requiring dedicated infrastructure and human resource. The 5-year survival is 59% for all stages combined and only 45% in patients with locally advanced inoperable head and neck cancer using current chemoradiation schedules. Chemotherapy agents administered in the induction or concurrent setting comprise of taxanes (Docetaxel, paclitaxel), platinum compounds (Cisplatin, carboplatin) and fluorouracil (TPF). For patients with advanced Head and neck squamous cell carcinoma (HNSCC), 3-weekly TPF regimen is the established standard induction chemotherapy (ICT) option based on overall survival benefit. However, TPF regimen is known to be associated with significant dose limiting toxicities which may impair tolerance and effectiveness of therapy. In this study we assessed the efficacy and toxicity of weekly vs. 3-weekly Docetaxel, Cisplatin, and Fluoro-uracil (TPF) induction chemotherapy in locally advanced Head and neck squamous cell carcinoma (LA-HNSCC). Methods: This was an open labeled randomized two arm study with 41 patients in the 3-weekly TPF arm and 41 patients in the weekly arm. Patients were randomized using numbers from a randomization software, data recorded, and results were analyzed. Results: The weekly group achieved far greater symptom relief than 3-weekly group (72 vs. 64%). The overall response rates were similar in both arms (ORR 75.6 and 73.1% in the weekly and 3-weekly groups, respectively). Renal toxicity was significantly lower in the weekly group as compared to 3 weekly arm post three cycles of chemotherapy (CrCl 91.49 ml/min vs. 76.67 ml/min, respectively). The weekly group had predominantly grade I and II neutropenia (19.5 and 17.1%, respectively) as compared to 3-weekly group where grade III and IV neutropenia (31 and 12%, respectively) was more prominent (p-0.003). Among non-hematological toxicities, mucositis, nausea/vomiting, and diarrhea in the weekly group were significantly lower when compared to 3-weekly group. Progression free survival was slightly higher in the weekly group (18 months) when compared to 3-weekly group (15 months) which was not statistically significant. Conclusion: Weekly induction with TPF had lower toxicity and similar efficacy as compared to 3-weekly regimen in locally advanced HNSCC patients. Myelosuppression, which was the most serious and common complication of 3-weekly TPF regimens was notably low using the weekly regimen. Our results suggest that weekly TPF regimen may be a safer and effective alternative to 3-weekly TPF for treatment of LA-HNSCC. To our knowledge this is the first study reporting the efficacy of weekly TPF regimen in LA-HNSCC till date.
Keywords: induction chemotherapy; locally advanced head and neck cancer; weekly 5-fluro uracil; weekly TPF; weekly cisplatin in head and neck cancer; weekly docetaxel.
Copyright © 2020 Tousif, Sarathy, Kumar and Naik.
Figures
References
-
- MCCOMB WS. Treatment of cancer of the head and neck. An Cir. (1961). 26:1–20. - PubMed
-
- Kulkarni MR. Head and neck cancer burden in India. Int J Head Neck Surg. (2013) 4:29–35. 10.5005/jp-journals-10001-1132 - DOI
-
- Hitt R, López-Pousa A, Martínez-Trufero J, Escrig V, Carles J, Rizo A, et al. . Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. (2005) 23:8636–45. 10.1200/JCO.2004.00.1990 - DOI - PubMed
LinkOut - more resources
Full Text Sources