LC3-Associated Phagocytosis (LAP): A Potentially Influential Mediator of Efferocytosis-Related Tumor Progression and Aggressiveness
- PMID: 32850405
- PMCID: PMC7422669
- DOI: 10.3389/fonc.2020.01298
LC3-Associated Phagocytosis (LAP): A Potentially Influential Mediator of Efferocytosis-Related Tumor Progression and Aggressiveness
Abstract
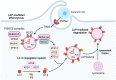
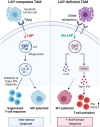
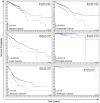
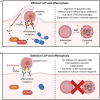
One aim of cancer therapies is to induce apoptosis of tumor cells. Efficient removal of the apoptotic cells requires coordinated efforts between the processes of efferocytosis and LC3-associated phagocytosis (LAP). However, this activity has also been shown to produce anti-inflammatory and immunosuppressive signals that can be utilized by live tumor cells to evade immune defense mechanisms, resulting in tumor progression and aggressiveness. In the absence of LAP, mice exhibit suppressed tumor growth during efferocytosis, while LAP-sufficient mice show enhanced tumor progression. Little is known about how LAP or its regulators directly affect efferocytosis, tumor growth and treatment responses, and identifying the mechanisms involved has the potential to lead to the discovery of novel approaches to target cancer cells. Also incompletely understood is the direct effect of apoptotic cancer cells on LAP. This is particularly important as induction of apoptosis by current cytotoxic cancer therapies can potentially stimulate LAP following efferocytosis. Herein, we highlight the current understanding of the role of LAP and its relationship with efferocytosis in the tumor microenvironment with a view to presenting novel therapeutic strategies.
Keywords: LAP; M2 macrophage activation; efferocytosis; tumor cell apoptosis; tumor immune response.
Copyright © 2020 Asare, Roscioli, Hurtado, Tran, Mah and Hodge.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

