CFTR Depletion Confers Hypersusceptibility to Mycobacterium fortuitum in a Zebrafish Model
- PMID: 32850470
- PMCID: PMC7396536
- DOI: 10.3389/fcimb.2020.00357
CFTR Depletion Confers Hypersusceptibility to Mycobacterium fortuitum in a Zebrafish Model
Abstract
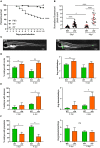
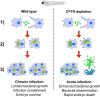
The Mycobacterium fortuitum complex comprises several closely related species, causing pulmonary and extra-pulmonary infections. However, there is very limited knowledge about the disease pathogenesis involved in M. fortuitum infections, particularly due to the lack of suitable animal models. Using the zebrafish model, we show that embryos are susceptible to M. fortuitum infection in a dose-dependent manner. Furthermore, zebrafish embryos form granulomas from as early as 2 days post-infection, recapitulating critical aspects of mycobacterial pathogenesis observed in other pathogenic species. The formation of extracellular cords in infected embryos highlights a previously unknown pathogenic feature of M. fortuitum. The formation of large corded structures occurs also during in vitro growth, suggesting that this is not a host-adapted stress mechanism deployed during infection. Moreover, transient macrophage depletion led to rapid embryo death with increased extracellular cords, indicating that macrophages are essential determinants of M. fortuitum infection control. Importantly, morpholino depletion of the cystic fibrosis transmembrane conductance regulator (cftr) significantly increased embryo death, bacterial burden, bacterial cords and abscesses. There was a noticeable decrease in the number of cftr-deficient infected embryos with granulomas as compared to infected controls, suggesting that loss of CFTR leads to impaired host immune responses and confers hypersusceptiblity to M. fortuitum infection. Overall, these findings highlight the application of the zebrafish embryo to study M. fortuitum and emphasizes previously unexplored aspects of disease pathogenesis of this significant mycobacterial species.
Keywords: CFTR; Mycobacterium fortuitum; cording; cystic fibrosis; granuloma; infection; pathogenesis; zebrafish.
Copyright © 2020 Johansen and Kremer.
Figures
Similar articles
-
CFTR Protects against Mycobacterium abscessus Infection by Fine-Tuning Host Oxidative Defenses.Cell Rep. 2019 Feb 12;26(7):1828-1840.e4. doi: 10.1016/j.celrep.2019.01.071. Cell Rep. 2019. PMID: 30759393
-
Control of Mycobacterium fortuitum and Mycobacterium intracellulare infections with respect to distinct granuloma formations in livers of BALB/c mice.Mem Inst Oswaldo Cruz. 2010 Aug;105(5):642-8. doi: 10.1590/s0074-02762010000500007. Mem Inst Oswaldo Cruz. 2010. PMID: 20835610
-
M. fortuitum-induced CNS-pathology: Deciphering the role of canonical Wnt signaling, blood brain barrier components and cytokines.Dev Comp Immunol. 2021 Sep;122:104111. doi: 10.1016/j.dci.2021.104111. Epub 2021 Apr 29. Dev Comp Immunol. 2021. PMID: 33933535
-
Mycobacterium abscessus, an Emerging and Worrisome Pathogen among Cystic Fibrosis Patients.Int J Mol Sci. 2019 Nov 22;20(23):5868. doi: 10.3390/ijms20235868. Int J Mol Sci. 2019. PMID: 31766758 Free PMC article. Review.
-
Topical antibacterial therapy for mycobacterial keratitis: potential for surgical prophylaxis and treatment.Clin Ther. 2004 Feb;26(2):191-6. doi: 10.1016/s0149-2918(04)90018-5. Clin Ther. 2004. PMID: 15038942 Review.
Cited by
-
Localized Infections with P. aeruginosa Strains Defective in Zinc Uptake Reveal That Zebrafish Embryos Recapitulate Nutritional Immunity Responses of Higher Eukaryotes.Int J Mol Sci. 2023 Jan 4;24(2):944. doi: 10.3390/ijms24020944. Int J Mol Sci. 2023. PMID: 36674459 Free PMC article.
-
Specificity of the innate immune responses to different classes of non-tuberculous mycobacteria.Front Immunol. 2023 Jan 18;13:1075473. doi: 10.3389/fimmu.2022.1075473. eCollection 2022. Front Immunol. 2023. PMID: 36741407 Free PMC article.
-
A fresh look at mycobacterial pathogenicity with the zebrafish host model.Mol Microbiol. 2022 Mar;117(3):661-669. doi: 10.1111/mmi.14838. Epub 2021 Nov 7. Mol Microbiol. 2022. PMID: 34714579 Free PMC article. Review.
-
Host Long Noncoding RNAs as Key Players in Mycobacteria-Host Interactions.Microorganisms. 2024 Dec 21;12(12):2656. doi: 10.3390/microorganisms12122656. Microorganisms. 2024. PMID: 39770858 Free PMC article. Review.
-
In Vitro and In Vivo Efficacy of NITD-916 against Mycobacterium fortuitum.Antimicrob Agents Chemother. 2023 Apr 18;67(4):e0160722. doi: 10.1128/aac.01607-22. Epub 2023 Mar 15. Antimicrob Agents Chemother. 2023. PMID: 36920188 Free PMC article.
References
-
- Alibaud L., Rombouts Y., Trivelli X., Burguière A., Cirillo S. L. G., Cirillo J. D., et al. . (2011). A Mycobacterium marinum TesA mutant defective for major cell wall-associated lipids is highly attenuated in Dictyostelium discoideum and zebrafish embryos. Mol. Microbiol. 80, 919–934. 10.1111/j.1365-2958.2011.07618.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

