2H/4H-Chromenes-A Versatile Biologically Attractive Scaffold
- PMID: 32850645
- PMCID: PMC7419998
- DOI: 10.3389/fchem.2020.00623
2H/4H-Chromenes-A Versatile Biologically Attractive Scaffold
Abstract
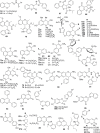
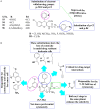
2H/4H-chromene (2H/4H-ch) is an important class of heterocyclic compounds with versatile biological profiles, a simple structure, and mild adverse effects. Researchers discovered several routes for the synthesis of a variety of 2H/4H-ch analogs that exhibited unusual activities by multiple mechanisms. The direct assessment of activities with the parent 2H/4H-ch derivative enables an orderly analysis of the structure-activity relationship (SAR) among the series. Additionally, 2H/4H-ch have numerous exciting biological activities, such as anticancer, anticonvulsant, antimicrobial, anticholinesterase, antituberculosis, and antidiabetic activities. This review is consequently an endeavor to highlight the diverse synthetic strategies, synthetic mechanism, various biological profiles, and SARs regarding the bioactive heterocycle, 2H/4H-ch. The presented scaffold work compiled in this article will be helpful to the scientific community for designing and developing potent leads of 2H/4H-ch analogs for their promising biological activities.
Keywords: 2H/4H-chromenes; biological activities; reaction mechanism; structure-activity relationship; synthetic strategies.
Copyright © 2020 Raj and Lee.
Figures
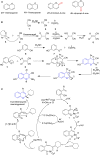

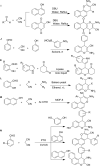
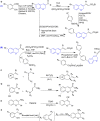

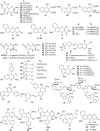

References
-
- Abdelatef S. A., El-Saadi M. T., Amin N. H., Abdelazeem A. H., Omar H. A., Abdellatif K. R. (2018). Design, synthesis and anticancer evaluation of novel spirobenzo [h] chromene and spirochromane derivatives with dual EGFR and B-RAF inhibitory activities. Eur. J. Med. Chem. 150, 567–578. 10.1016/j.ejmech.2018.03.001 - DOI - PubMed
-
- Abd-El-Aziz A., El-Ghezlani E., Elaasser M., Afifi T., Okasha R. (2020). First example of cationic cyclopentadienyliron based chromene complexes and polymers: synthesis, characterization, and biological applications. J. Inorg. Organomet. Polym. Mater. 30, 131–146. 10.1007/s10904-019-01295-w - DOI
-
- Abd-El-Aziz A. S., Alsaggaf A. T., Okasha R. M., Ahmed H. E., Bissessur R., Abdelghani A. A., et al. (2016). Antimicrobial and antitumor screening of fluorescent 5, 7-dihydroxy-4-propyl-2h-chromen-2-one derivatives with docking studies. Chem. Select 1, 5025–5033. 10.1002/slct.201600789 - DOI
-
- Abrouki Y., Anouzla A., Loukili H., Chakir A., Idrissi M., Abrouki A., et al. (2013). Aqua mediated synthesis of substituted 2-amino-chromenes catalyzed by expanded perlite. Am. J. Biol.Chem. Pharm. Sci. 1, 28–34.
-
- Afifi T. H., Okasha R. M., Ahmed H. E., Ilaš J., Saleh T., Abd-El-Aziz A.S. (2017a). Structure-activity relationships and molecular docking studies of chromene and chromene based azo chromophores: a novel series of potent antimicrobial and anticancer agents. EXCLI J. 16, 868. 10.2174/1570179414666170519150520 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

