High-Level Expression and Biochemical Properties of A Thermo-Alkaline Pectate Lyase From Bacillus sp. RN1 in Pichia pastoris With Potential in Ramie Degumming
- PMID: 32850721
- PMCID: PMC7396651
- DOI: 10.3389/fbioe.2020.00850
High-Level Expression and Biochemical Properties of A Thermo-Alkaline Pectate Lyase From Bacillus sp. RN1 in Pichia pastoris With Potential in Ramie Degumming
Abstract
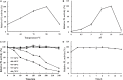
Pectate lyases play an essential role in textiles, animal feed, and oil extraction industries. Pichia pastoris can be an ideal platform for pectate lyases production, and BspPel (a thermo-alkaline pectate lyase from Bacillus sp. RN1) was overexpressed by combined strategies, reaching 1859 U/mL in a 50 L fermentator. It displayed the highest activity at 80°C, and maintained more than 60% of the activity between 30 and 70°C for 1 h. It showed an optimal pH of 10.0, and exhibited remarkable stability over a wider pH range (3.0-11.0), retaining more than 80.0% of enzyme activity for 4 h. The K m and k cat of BspPel on PGA (polygalacturonic acid) was 2.19 g L-1 and 116.1 s-1, respectively. The activity was significantly enhanced by Ca2+, Mn2+, and Cu2+, and a slight increase was observed with the addition of Ba2+ and Mg2+. Scanning electron microscope was used to show the degumming efficiency of BspPel on ramie fibers. The loss weight was 9.2% when treated with crude enzyme supernatant and 20.8% when treated with the enzyme-chemical method, which was higher than the 14.2% weight loss in the positive control treated with 0.5% (w/v) NaOH alone. In conclusion, BspPel could be a good candidate for the ramie degumming industry.
Keywords: Pichia pastoris; biochemical properties; high-level expression; pectate lyase; ramie degumming.
Copyright © 2020 Zheng, Zhang, Liu, Li, Lin and Liang.
Figures





Similar articles
-
The alkaline pectate lyase PEL168 of Bacillus subtilis heterologously expressed in Pichia pastoris is more stable and efficient for degumming ramie fiber.BMC Biotechnol. 2013 Mar 19;13:26. doi: 10.1186/1472-6750-13-26. BMC Biotechnol. 2013. PMID: 23510095 Free PMC article.
-
Structure of an Alkaline Pectate Lyase and Rational Engineering with Improved Thermo-Alkaline Stability for Efficient Ramie Degumming.Int J Mol Sci. 2022 Dec 29;24(1):538. doi: 10.3390/ijms24010538. Int J Mol Sci. 2022. PMID: 36613981 Free PMC article.
-
Cloning, evaluation, and high-level expression of a thermo-alkaline pectate lyase from alkaliphilic Bacillus clausii with potential in ramie degumming.Appl Microbiol Biotechnol. 2017 May;101(9):3663-3676. doi: 10.1007/s00253-017-8110-2. Epub 2017 Feb 9. Appl Microbiol Biotechnol. 2017. PMID: 28184988
-
A novel pectate lyase with high specific activity from Bacillus sp. B58-2: Gene cloning, heterologous expression and use in ramie degumming.Enzyme Microb Technol. 2024 Apr;175:110395. doi: 10.1016/j.enzmictec.2024.110395. Epub 2024 Jan 16. Enzyme Microb Technol. 2024. PMID: 38237242
-
Origins and features of pectate lyases and their applications in industry.Appl Microbiol Biotechnol. 2020 Sep;104(17):7247-7260. doi: 10.1007/s00253-020-10769-8. Epub 2020 Jul 14. Appl Microbiol Biotechnol. 2020. PMID: 32666183 Review.
Cited by
-
Green Process: Improved Semi-Continuous Fermentation of Pichia pastoris Based on the Principle of Vitality Cell Separation.Front Bioeng Biotechnol. 2021 Nov 30;9:777774. doi: 10.3389/fbioe.2021.777774. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34917600 Free PMC article.
-
Structural Homology Fails to Predict Secretion Efficiency in Pichia pastoris: Divergent Responses of Architecturally Similar scFvs to Multi-Parametric Genetic Engineering.Int J Mol Sci. 2025 May 21;26(10):4922. doi: 10.3390/ijms26104922. Int J Mol Sci. 2025. PMID: 40430062 Free PMC article.
-
Improvement of optimum pH and specific activity of pectate lyase from Bacillus RN.1 using loop replacement.Front Bioeng Biotechnol. 2023 Jul 4;11:1242123. doi: 10.3389/fbioe.2023.1242123. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37469444 Free PMC article.
-
Metagenomic and proteomic analysis of bacterial retting community and proteome profile in the degumming process of kenaf bast.BMC Plant Biol. 2022 Nov 5;22(1):516. doi: 10.1186/s12870-022-03890-5. BMC Plant Biol. 2022. PMID: 36333799 Free PMC article.
-
Modification and application of highly active alkaline pectin lyase.AMB Express. 2022 Oct 9;12(1):130. doi: 10.1186/s13568-022-01472-0. AMB Express. 2022. PMID: 36210372 Free PMC article.
References
-
- Calafell M., Klug-Santner B., Gübitz G., Garriga P. (2005). Dyeing behaviour of cotton fabric bioscoured with pectate lyase and polygalacturonase. Color. Technol. 121 291–297. 10.1111/j.1478-4408.2005.tb00371.x - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

