Rhodnius, Golden Oil, and Met: A History of Juvenile Hormone Research
- PMID: 32850806
- PMCID: PMC7426621
- DOI: 10.3389/fcell.2020.00679
Rhodnius, Golden Oil, and Met: A History of Juvenile Hormone Research
Abstract
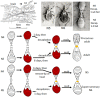
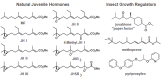
Juvenile hormone (JH) is a unique sesquiterpenoid hormone which regulates both insect metamorphosis and insect reproduction. It also may be utilized by some insects to mediate polyphenisms and other life history events that are environmentally regulated. This article details the history of the research on this versatile hormone that began with studies by V. B. Wigglesworth on the "kissing bug" Rhodnius prolixus in 1934, through the discovery of a natural source of JH in the abdomen of male Hyalophora cecropia moths by C. M. Williams that allowed its isolation ("golden oil") and identification, to the recent research on its receptor, termed Methoprene-tolerant (Met). Our present knowledge of cellular actions of JH in metamorphosis springs primarily from studies on Rhodnius and the tobacco hornworm Manduca sexta, with recent studies on the flour beetle Tribolium castaneum, the silkworm Bombyx mori, and the fruit fly Drosophila melanogaster contributing to the molecular understanding of these actions. Many questions still need to be resolved including the molecular basis of competence to metamorphose, differential tissue responses to JH, and the interaction of nutrition and other environmental signals regulating JH synthesis and degradation.
Keywords: Hyalophora cecropia; Manduca sexta; Methoprene-tolerant; Rhodnius prolixus; juvenile hormone.
Copyright © 2020 Riddiford.
Figures





References
-
- Aminoff M. J. (2017). The life and legacy of Brown-Séquard. Brain 140 1525–1532. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

