Inhibiting Heat Shock Protein 90 Protects Nucleus Pulposus-Derived Stem/Progenitor Cells From Compression-Induced Necroptosis and Apoptosis
- PMID: 32850811
- PMCID: PMC7427414
- DOI: 10.3389/fcell.2020.00685
Inhibiting Heat Shock Protein 90 Protects Nucleus Pulposus-Derived Stem/Progenitor Cells From Compression-Induced Necroptosis and Apoptosis
Abstract
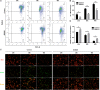
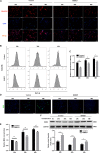
Nucleus pulposus-derived stem/progenitor cells (NPSCs) provide novel prospects for the regeneration of degenerated intervertebral disc (IVD). Nevertheless, with aging and degeneration of IVD, the frequency of NPSCs markedly decreases. Excessive cell death could be the main reason for declined frequency of NPSCs, however, the exact mechanisms remain elusive. Thus, the present study was undertaken to explore the mechanisms of compression-induced NPSCs death, and the effects of heat shock protein 90 (HSP90) on NPSCs survival. Here, we found that compression could trigger receptor-interacting protein kinase 1 (RIPK1)/receptor-interacting protein kinase 3 (RIPK3)/mixed lineage kinase domain-like protein (MLKL)-mediated necroptosis of NPSCs. Furthermore, we found that elevated expression of HSP90 was involved in compression-induced NPSCs death, and inhibiting HSP90 could dramatically attenuate compression-induced necroptosis of NPSCs via regulating the expression and activity of RIPK1/RIPK3/MLKL, and alleviating the mitochondrial dysfunction (mitochondrial membrane potential loss and ATP depletion) and oxidative stress [production of mitochondrial reactive oxygen species (ROS), cellular total ROS and malondialdehyde, and downregulation of superoxide dismutase 2]. Besides necroptosis, compression-induced apoptosis of NPSCs was also attenuated by HSP90 inhibition. In addition, we found that enhanced expression of HSP70 contributed to the cytoprotective effects of inhibiting HSP90. More encouragingly, our results demonstrated that inhibiting HSP90 could also mitigate the exhaustion of NPSCs in vivo. In conclusion, RIPK1/RIPK3/MLKL-mediated necroptosis participates in compression-induced NPSCs death. Furthermore, targeting HSP90 to simultaneously inhibit necroptosis and apoptosis of NPSCs might be an efficient strategy for preventing the death of NPSCs, thus rescuing the endogenous repair capacity of NP tissue.
Keywords: apoptosis; compression; heat shock protein 90; intervertebral disc degeneration; necroptosis; nucleus pulposus-derived stem/progenitor cells.
Copyright © 2020 Hu, Zhang, Liu, Wang, Chen, Lv, Shi, Ma, Wang, Wu and Shao.
Figures








Similar articles
-
RIPK1/RIPK3/MLKL-mediated necroptosis contributes to compression-induced rat nucleus pulposus cells death.Apoptosis. 2017 May;22(5):626-638. doi: 10.1007/s10495-017-1358-2. Apoptosis. 2017. PMID: 28289909
-
Activation of HSP70 impedes tert-butyl hydroperoxide (t-BHP)-induced apoptosis and senescence of human nucleus pulposus stem cells via inhibiting the JNK/c-Jun pathway.Mol Cell Biochem. 2021 May;476(5):1979-1994. doi: 10.1007/s11010-021-04052-1. Epub 2021 Jan 28. Mol Cell Biochem. 2021. PMID: 33511552
-
Critical contribution of RIPK1 mediated mitochondrial dysfunction and oxidative stress to compression-induced rat nucleus pulposus cells necroptosis and apoptosis.Apoptosis. 2018 Jun;23(5-6):299-313. doi: 10.1007/s10495-018-1455-x. Apoptosis. 2018. PMID: 29705943
-
Mechanisms of endogenous repair failure during intervertebral disc degeneration.Osteoarthritis Cartilage. 2019 Jan;27(1):41-48. doi: 10.1016/j.joca.2018.08.021. Epub 2018 Sep 19. Osteoarthritis Cartilage. 2019. PMID: 30243946 Review.
-
Spotlight on necroptosis: Role in pathogenesis and therapeutic potential of intervertebral disc degeneration.Int Immunopharmacol. 2024 Sep 10;138:112616. doi: 10.1016/j.intimp.2024.112616. Epub 2024 Jul 2. Int Immunopharmacol. 2024. PMID: 38959544 Review.
Cited by
-
Integrating bioinformatics and experimental validation to Investigate IRF1 as a novel biomarker for nucleus pulposus cells necroptosis in intervertebral disc degeneration.Sci Rep. 2024 Dec 3;14(1):30138. doi: 10.1038/s41598-024-81681-8. Sci Rep. 2024. PMID: 39627301 Free PMC article.
-
HSP70 attenuates compression-induced apoptosis of nucleus pulposus cells by suppressing mitochondrial fission via upregulating the expression of SIRT3.Exp Mol Med. 2022 Mar;54(3):309-323. doi: 10.1038/s12276-022-00745-9. Epub 2022 Mar 25. Exp Mol Med. 2022. PMID: 35338257 Free PMC article.
-
HSPA8 acts as an amyloidase to suppress necroptosis by inhibiting and reversing functional amyloid formation.Cell Res. 2023 Nov;33(11):851-866. doi: 10.1038/s41422-023-00859-3. Epub 2023 Aug 14. Cell Res. 2023. PMID: 37580406 Free PMC article.
-
Investigating the characteristics of mild intervertebral disc degeneration at various age stages using single-cell genomics.Front Cell Dev Biol. 2024 Jul 2;12:1409287. doi: 10.3389/fcell.2024.1409287. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39015652 Free PMC article.
-
Programmed cell death in stem cell-based therapy: Mechanisms and clinical applications.World J Stem Cells. 2021 May 26;13(5):386-415. doi: 10.4252/wjsc.v13.i5.386. World J Stem Cells. 2021. PMID: 34136072 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

