Mediastinal Lymphadenopathy, Class-Switched Auto-Antibodies and Myocardial Immune-Complexes During Heart Failure in Rodents and Humans
- PMID: 32850816
- PMCID: PMC7426467
- DOI: 10.3389/fcell.2020.00695
Mediastinal Lymphadenopathy, Class-Switched Auto-Antibodies and Myocardial Immune-Complexes During Heart Failure in Rodents and Humans
Abstract
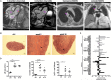
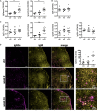
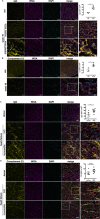
Mediastinal lymphadenopathy and auto-antibodies are clinical phenomena during ischemic heart failure pointing to an autoimmune response against the heart. T and B cells have been convincingly demonstrated to be activated after myocardial infarction, a prerequisite for the generation of mature auto-antibodies. Yet, little is known about the immunoglobulin isotype repertoire thus pathological potential of anti-heart auto-antibodies during heart failure. We obtained human myocardial tissue from ischemic heart failure patients and induced experimental MI in rats. We found that anti-heart autoimmunity persists during heart failure. Rat mediastinal lymph nodes are enlarged and contain active secondary follicles with mature isotype-switched IgG2a B cells. Mature IgG2a auto-antibodies specific for cardiac antigens are present in rat heart failure serum, and IgG and complement C3 deposits are evident in heart failure tissue of both rats and human patients. Previously established myocardial inflammation, and the herein provided proof of B cell maturation in lymph nodes and myocardial deposition of mature auto-antibodies, provide all the hallmark signs of an established autoimmune response in chronic heart failure.
Keywords: adaptive immune system; anti-heart; auto-antibodies; autoimmunity; chronic heart failure; myocardial infarction.
Copyright © 2020 Sintou, Mansfield, Iacob, Chowdhury, Narodden, Rothery, Podovei, Sanchez-Alonso, Ferraro, Swiatlowska, Harding, Prasad, Rosenthal, Gorelik and Sattler.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

